Introduction
Epoxy polymer composites with carbon fiber as reinforcement have an extensive range of applications in many industrial fields as: wind turbines [1], construction [2], aeronautics [3] and aerospace [4] due to their favorable strength-to-weight and stiffness-to-weight ratios [5], as well as their high thermal stability and excellent corrosion resistance [6]. The performance of fiber-reinforced composites is affected by the properties of the constituent materials and the load transfer capacity from the matrix to the reinforcement, this latter feature determined by the interfacial chemical interactions between fiber and matrix [5]. However, since the present interactions are poor due to the surface inertness of carbon fiber [7], the efficiency of composite is limited.
In order to achieve a greater performance, there are different approaches to modify the surface of the fiber such as polymeric coating [8], thermal treatment [9], chemical partial oxidation [10], plasma treatment [11], increase of the surface roughness of carbon fibers [12], or integration of nanoparticles as fillers into the surface of the reinforcement [13]. Besides the mentioned approaches, an alternative technique that has recently emerged is the dispersion of nanofillers into the polymer matrix [5]. Graphene is a candidate to be applied as nanofiller because of its superior electrical, thermal and mechanical properties [14]. However, the use of graphene is limited by the lack of an effective method for large-scale production [15], by its high tendency to agglomerate via Van der Waals interactions and by its flammability [16,17]. Hence, Graphene Oxide (GO) and reduced graphene oxide (rGO) are used instead of pristine graphene because both (of them) contain functional groups such as carboxylic and hydroxyl ones that facilitate their dispersion in epoxy matrix [18]. Functional groups, can interact with some molecular structures inside resins composition, and hence, improving its cohesion. The main difference between GO and rGO is that, while the former has more functional groups, the latter is more similar to pris- tine graphene [9]. Several studies have been carried out to determine effects of applying GO or rGO as nanofiller [19] achieved improvements in flexural strength by 66 %, flexural modulus by 72 %, and inter laminar shear strength by 25% at 0.3 wt % of GO included in the carbon fiber/epoxy composite [5] employed electrospraying for the deposition of 0.05 wt % graphene sheets onto carbon fiber and attained enhancements in the flexural strength and modulus by about 64 % and 85 %, respectively [14].
Dispersed 0.2 wt % rGO to epoxy resin and attained an increase of glass transition temperature by nearly 11°C, and enhanced its quasi-static fracture toughness by about 52% [20] added 1.5 vol % GO to the epoxy matrix and increased its tensile strength from 7 MPa to 13 MPa, the Youngs modulus improved from 115 MPa to 206 MPa, the maximum applied load from 126 N to 234 N and the elongation at break value jumped from 38 % to 55 %. Noticeably the number of cycles to failure for the case of GO, directly spray-coated onto the glass fibers, was about 8 times greater than when the GO was uniformly dispersed in the resin [21,22] introduced 5 wt % of GO sheets dispersed in the fiber sizing onto the surface of carbon fibers and achieved significant enhancement of Interfacial Shear Strength (IFSS), Inter laminar Shear Strength (ILSS) and tensile properties [23] combined the graphene nanopowder with a dispersing agent before mixing it with the epoxy resin to maximize GO dispersion, and the fracture toughness of the carbon fiber/GO/epoxy resin composite improved an 11.4 % in comparison to conventional composite without GO.
The synthesis of the catalyzer for the dehydrogenation of DMAB in ambient temperature, prepared from three monodispersed metallic nano compound (Pd, Ru, Mi) in GO. (PdRuNi@GO). The GO allows the catalyzer to be chemically stable, to be a conductor and to have a high catalytic activity, thus obtaining a great efficiency in TOF for the dehydrogenation catalyzers. Functionalized GO with thiocarbomide as a Rh-Pt monodispersed NPs support. (RhPt/TC@GO NPs) for the Kroevenagel condensation of amil aldehyde with malononitril. Very good catalytic activity for the reactions is obtained. This can be used in different organic reactions, making them faster. Compound formed by polyaniline-RGO with Pt (Pt@rGO-PANi) to improve the oxidation reaction of the alcohol (catalyzer).
Material formed by monodispersed Pt and GO NPs synthesized with microwave method (Mw-PtNPs@GO). To be used as counter electrode in dye sensitized solar cells, which produce electricity via photo-electrochemistry. It changes light energy into electricity with better conversion and efficiency ~7.96%. They have a high electro-catalytic activity. In the present work, Thermogravimetric Analysis (TGA), X-Ray Photoelectron Spectroscopy (XPS), Scanning Electron Microscopy (SEM) and tensile and flexural tests are utilized to study the influence of GO/rGO as reinforcing fillers in mechanical performance of nano-modified carbon fiber/epoxy resin composites. Carbon Fiber has demonstrated in the majority of cases of its application as composites base, a lot of difficulties to reach the close contact with resins used. The general approach offered by the majority of works [20] never states this fact. The use of nano-particles is the most promising technique to try to improve its physical final properties, as can be seen in our experimental results. The strength remains, or indeed increases lightly, but flexibility is really improved. The use of these kinds of nano-fillers, due to its three-dimension capability to, chemically, interact with resin, allows getting closer the tailor-made approach. Just depending on the chemical constitution of the resin, different amounts of different nano-fillers, with different chemical groups available can be used to modify only the desired characteristics of the final product, without altering its principal mechanical behavior
Materials
Epoxy resins
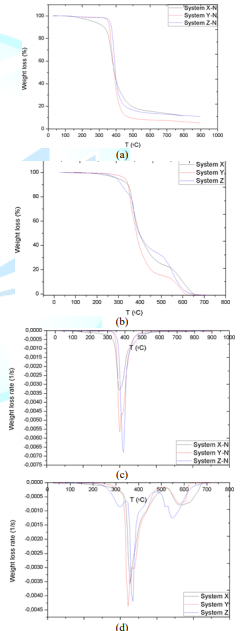
Epoxy resins are compounds containing several cyclic ethers with three-atom rings per molecule; the backbones of commercial resins are usually aliphatic, cycloaliphatic or aromatic. The preparation process of epoxy resins has a great influence on their functionalities, and treatment with crosslinking agents produces three-dimensional insoluble thermoset polymers. These curing agents can be Lewis acids/bases, amines or anhydrides. Three commercial epoxy resins with their correspondent hardeners denominated as systems X, Y and Z were employed as the matrix of the composites. To identify the characterizing of cross-linked resins, thermogravimetric analysis was utilized; matrix samples were put through thermal decomposition under nitrogen atmosphere from 25-900°C with a heating rate of 20°C/min. TGA and DTG plots (Figures 1a and 1b) obtained hint that systems X and Y are quite similar since their resins are Biphenol Epoxy (BP) and Tetramethyl Biphenyl Epoxy (TMBP), respectively, and both of them share Methyl Cyclohexane-1.2-Dicarboxylic Anhydride (MCHDA) as the crosslinking agent [24]. In the particular case of matrix system Z, it is composed by bisphenol epoxy (DGEBA) and polyoxypropylene diamine (D230) [25].
The commercial form of the mentioned epoxy resins may include rheology modifiers; as a re Ma lt of adding these additives, BP, TMBP and DEGBA viscosity at 20°C are 1950 ± 200, 3240 ± 560 and 9800 ± 1000 cps, accordingly. The main and immediately noticeable difference between the systems mentioned is the curing process; whereas resins X and Y require 8 h at 80°C after sitting for 24 h at room temperature to complete their crosslinking reaction, resin Z needs 16 h at 60°C after the same precuring conditions. Figures 1c and 1d present the TGA of the matrix systems performed under air atmosphere between the same range of temperatures and heating rate as the method described above. This analysis was carried out to get an insight into the oxidation behavior of cross-linked resins. Two common thermal degradation stages can be distinguished in all systems, albeit they manifest different inflection points and intensities. In the particular case of system Z, there is an additional stage of weak intensity indicating once again that this matrix is the most distinctive one.
Carbon fiber
Carbon fibers are well known for having a high superficial inertness; this feature is due to their scarce functional groups, as illustrated in the Figure 2. The carbon fiber selected as reinforcement of the composite is characterized by its twill style weave and an area weight of 300 g/cm2.
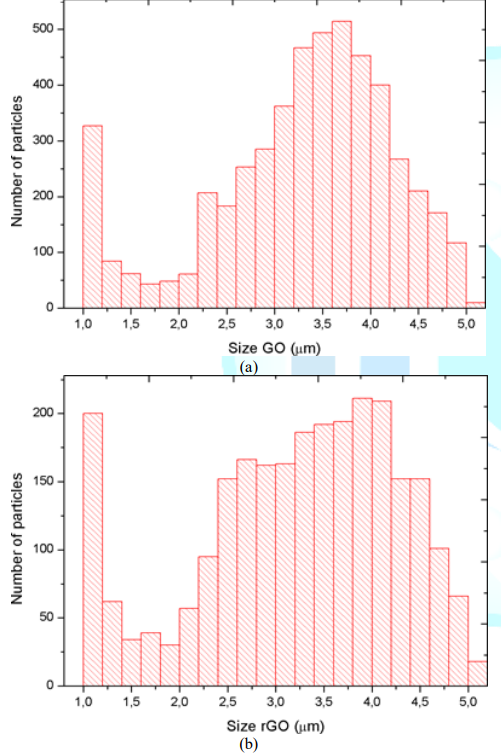
Figure 2: Size distribution of particles of the fillers: (a) GO particles; (b) rGO particles
GO/rGO
Reinforcing fillers employed to achieve modification of interfacial interactions between carbon fiber and epoxy resins are 99 % pure flake-shaped GO/rGO nanopowder. Particle size distribution of GO/rGO is shown in Figure 3 GO particles are primarily distributed between 3 - 4 µm and a remarkable quantity of particles is of 1 µm while the number of particles with other sizes is considerably lower. A similar tendency is present in rGO size distribution, which has a high scope including sizes of 1 µm and between 2.5-4.5 µm. XPS was the characterization technique employed by the GO/rGO supplier to identify carbon and oxygen functional groups present on the surface of the particles. The C1s scan applied involves the electron transition from carbon-oxygen atoms of different atomic configurations and their shape relies on their atomic densities. The deconvolution of GO C1s spectra shown in Figure 3a is splitting into 6 peaks: C-C sp2 Monfared Zanjani et al. at 283.9 eV, C-C in graphitic type at 284.7 eV, C-O single bound at 286.5 eV, C=O double bond at 287.7 eV, O-C=O at 288.9 and the shake-up satellite at 291 eV. The deconvolution of rGO C1 spectra shown in Figure 3b is split into 4 peaks; C-C in graphitic type at 284.6 eV, C-O single bond at 286.4 eV, C=O double bond at 287.7 eV and O-C=O at 288.7 eV.
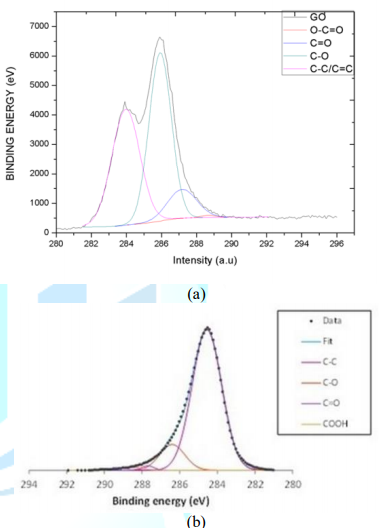
Figure 3: XPS characterization of fillers: (a) GO; (b) rGO
Equipment
CY-500 Sonicator from Optic Ivienm System and T-10 basic ULTRA-TURRAX from IKA were employed to homogenize the epoxy resin-GO/rGO systems. An Olympus microscope model BX43 and Advanced Image Analysis and Nanoparticles Size Identification (APSI) software were utilized to check the particle size of GO/rGO once dispersed into the matrix. Model JSM-5610 by JEOL scanning Electron Microscope was employed to obtain high-resolution images of prepared sample surfaces. Thermogravimetric Analysis was performed with a TGA/SDTA851e Analyzer from Mettler Toledo, an instrument run by STARe Software. An electro-mechanical material testing machine fitted with a ball-screw drive from E series by Zwick Roell group was employed to determine the mechanical performance of the composites.
Experimental method
Preparation of GO/rGO modified carbon fiber/epoxy composites
To modify the epoxy resins, 0.3 wt % of GO was dispersed in the matrix by ultrasonication for 30 min achieving a homogeneous system and then was mixed with the crosslinking agent. The mixture was applied to the fiber by manual impregnation using the wet lay-up technique. A similar process was followed to disperse rGO in the resin, but an additional homogenizing process at a speed of about 20500 rpm with Ultra Turrax was required to get a nano heterogeneous system before incorporating the crosslinking agent. The curing conditions of each epoxy resin/hardener systems are specified in the materials section above and the preparation process is depicted schematically in the Figure 4, where the homogenizing process refers to ultrasonication plus Ultra Turrax to disperse rGO, or only the first procedures when incorporating GO in the matrix.
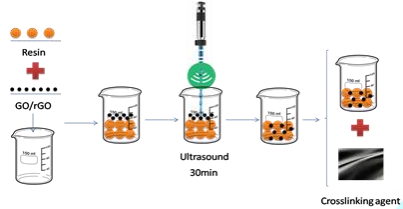
Figure 4: Preparation process scheme.
Determination of mechanical properties of the composites
Tensile and bending tests were performed on the obtained composites to determine changes in their mechanical properties. Tensile tests were performed in accordance with specifications designated in ISO 527-1:2012. Test specimens taken from flat areas of composites had a width of 15 ± 5 mm, an overall length of 250 mm and a thickness less than or equal to 1 mm. The tests were conducted at a speed of 2 mm/min. Three-point bending tests were conducted at a speed of 1 mm/min on specimens which dimensions were 100 × 15 × 2 mm, according to ISO 14125:1998.
TGA Characterization
Thermogravimetric analysis was applied to characterize raw carbon fiber, epoxy resins and elaborated composites. Samples of about 10 mg were placed in aluminum oxide crucibles and then placed on the microbalance of the analyzer. Thermal degradation experiments were performed from 25° C to 1025°C under air atmosphere at a heating rate of 20°C/min [26-27].
SEM samples preparation
Test samples utilized in tensile tests were analyzed afterwards with SEM. Specimens were taken as close as possible to the fracture zone. Clean ruptures were vertically placed in the sample holder while transverse cracks were horizontally disposed. A thin film of gold was deposited via 30min sputtering process onto the specimen before inserting the sample holder into the chamber of the microscope.
Results and Discussion
Mechanical properties
Youngs Modulus, rupture stress and elongation to failure results are information provided by the tensile test while the flexural stress result is given by the bending test; variations of mechanical properties due to the incorporation of reinforcing fillers are collected in Table 1. Many observations can be pointed out by data interpretation: whereas Youngs Modulus of X-CF composites experience a great boost by adding GO/rGO. systems with resins Y and Z not only are barely affected by the incorporation of GO (more possible molecular interactions due to the presence of more functional groups) but also undergo a slight reduction of the value of elastic modulus because of the presence of rGO (less interactions due to functional groups). In regard to the rupture stress, the values of X-GO/rGO-CF and Y-GO/rGO-CF are over two times higher than the composite without fillers, but the Z-CF composites show the same tendency as elastic modulus. As for the elongation to failure, GO/rGO has a positive impact on X-CF systems, but no significant variations are detected in Y-CF and Z-CF composites. Bending stress result are also boosted in X-GO/rGO-CF systems, but the opposite effect is observed in Y-GO/rGO-CF composites, and in the case of Z-CF ones, only rGO seems to improve this property.
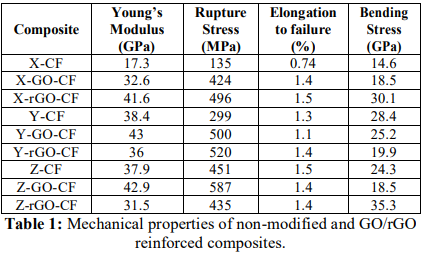
Table 1: Mechanical properties of non-modified and GO/rGO reinforced composites.
The observations made above suggest that the dispersion of 0.3 wt % of GO in the epoxy resin before the impregnation process certainly enhances the mechanical properties of the composites, probably due to the existence of molecular interactions reacted during the dispersion. Adding the same percentage of rGO in the system generally results in performance deterioration caused by such factors as poor dispersion of rGO into epoxy, agglomeration and undesired interaction of GO with a sizing agent [28]. In the particular case of X-CF composites, rGO also improves the mechanical performance, which suggests that resin X admits a higher quantity of nanoparticles than resins Y and Z before reaching the saturation point [19], where rGO no longer acts as reinforcing filler.
Scanning Electron Microscopy
Fracture surface of samples observed by SEM are shown in Figure 5. Whereas non- modified composites Figure 5a crack transversely leaving some fibers damaged and some others untouched, test samples of GO modified composites Figure 5b break into two halves with a clean rupture. Moreover, Figure 5b shows interlaminar distribution coinciding with the impregnation method. Nanoparticles present are shown in Figure 6. GO and rGO modified resins show a higher density of particles compared to those without reinforcing fillers. Changes in mechanical properties can be observed in the SEM images, where non-modified specimens Figure 6 and modified composites without enhancement of mechanical properties. Figure 6 manifest a bad adhesion (weak or null interaction) between fibers and epoxy matrix, which causes the occurrence of the rupture in the interfacial zone. In regard to the composites that experienced a remarkable boost in their mechanical performance Figure 6, the epoxy matrix remains attached to the fibers when breaking, what hints which at a notable adhesion between components.
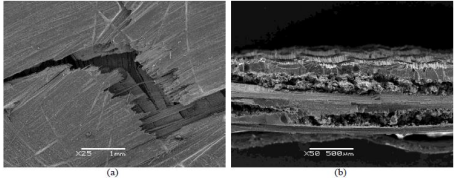
Figure 5: SEM images of fractures caused by applying tensile tests on Z-CF composites.
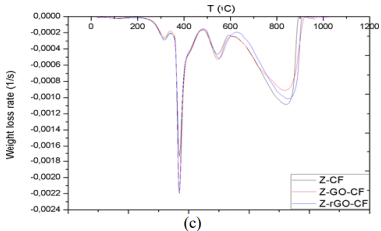
With the aim to getting more detailed information about the effects of reinforced composite with GO/rGO particles, first derivative of the TGA curves was applied to obtain DTG plots, the results of which are compiled in Figure 8. The graph allows comparing the variation of the thermal degradation phases selected, reflecting the level of order that interactions should create between fiber and resin, thanks to the existence of new interfaces promoted by GO/rGO presence in the previous dispersion and posterior cooling process. In Figure 7, peaks are described with the parameters below:
Width (W): horizontal distance from one maximum to the next one which indicates the variety of interactions ruptured in a degradation stage. Height (H): also known as intensity is the vertical distance from the highest maximum to the minimum and expresses the quantity of chemical bonds de- composed.
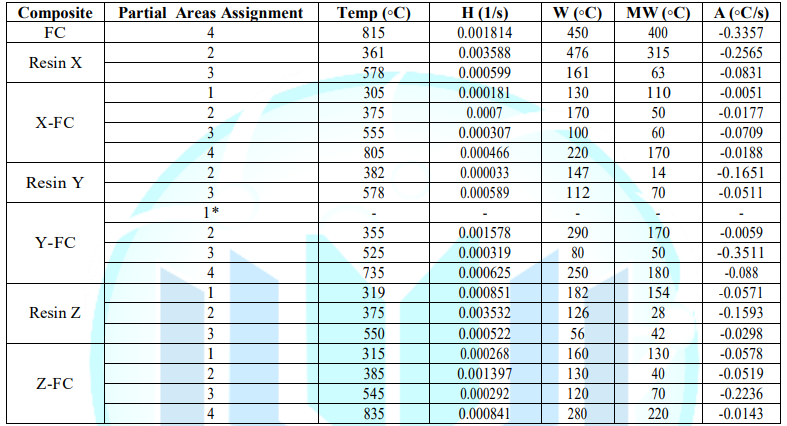
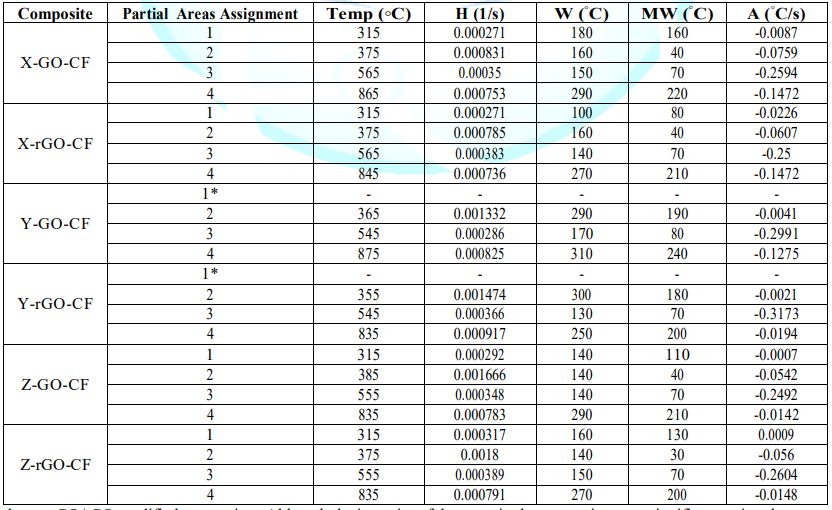
Area (A) and Mean Width (MW): represent the relation between variety and quantity of interactions. Additionally, the Advanced Analysis TGA (AATGA) software was developed to get a precise analysis of DTG plots. Data obtained from the program a non-modified composite are gathered in the Table 2 and those GO/rGO composites are collected in Table 3.
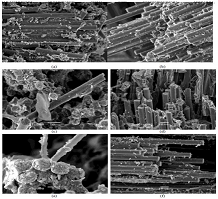
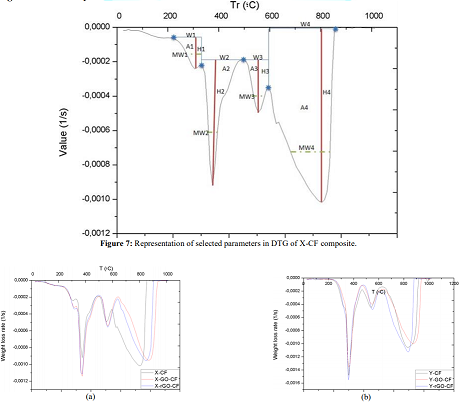
Figure 7: Representation of selected parameters in DTG of X-CF composite.
Thermogravimetric Analysis
The first remarkable difference when comparing the tables above is that the area increases of modified X-CF systems are considerably greater than to those of modified Y-CF and Z-CF composites. More specifically, whereas that the mean width of the partial areas have broadened in modified X-CF and Y-CF composites, no relevant variation is observed in Z-CF composites with fillers. TGA and DTG curves of the carbon fiber are shown in the Figure 9. Graphic interpretation indicates that the onset and offset temperatures of main phase of the thermal degradation are approximates 572 and 874°C, respectively; moreover, the first derivative of the TGA curve determines 815°C as the temperature where the maximum rate of mass loss takes place. DTG plots of matrix systems are shown in Figure 1d with their parameters collected in Table 2. Whereas matrices X and Y present two main degradation stages assigned as partial areas 2 and 3, an additional phase known as partial area 1 can be identified in system Z. With regard to DTG curves of obtained composites, four zones that correspond to different rupture of interactions or chemical bonds of the structure formed can be distinguished independently of the matrix system applied:
• Partial Area 1, around 250°C: the decomposition intensity is quite weak, which indicates the presence of a few interactions between matrix and reinforcement. Whereas this area is noticeable in composites with resins X and Z, Y-CF, Y-GO/rGO-CF only exhibits a slight shoulder. The incorporation of fillers also modifies the stage slightly.
• Partial Area 2, around 380-400°C: the high intensity of this stage suggests rupture of chemical bonds that belongs to the cross-linked resin, since this signal is not present in the DTG of carbon fiber. The inclusion of GO/rGO has no noticeable influence in this decomposition zone.
• Partial Area 3, around 500°C: the low intensity and the absence of this signal in carbon fiber suggest that it corresponds to the rupture of interactions between the residual structure of resin and carbon fiber. Although a slight shift can be observed in the temperature where maximum rate of weight loss occurs, this stage is scarcely modified by the presence of GO/rGO.
• Partial Area 4, around 800°C: this phase corresponds to the thermal degradation of the carbon fiber. The increase of the offset temperature of the decomposition indicates existence of intra fiber interactions due to the presence of fillers.
The use of active fillers (chemical structures that allow the creation of transversal or lateral interactions with the resin and core substrate) is more important when high performance materials are to be obtained. Mechanical performance enhancements are directly linked to the variations of DTG; a variation of about 11 % in the main area involves an increase in the properties, in the case where there is no change in the main area, a secondary area greater than half of a main one experiencing the same modification it is also translated into a boost of properties. Another pattern related to bending stress was that modified composites are stiffer than non-modified ones if there is a decrease greater or equal to 10 % in the main area; otherwise, the composites are more flexible.
Conclusion
Throughout the article, several facts have been stated clearly from the experimental results obtained: When working with composite materials compressed of carbon fiber and epoxy resins, the structure of hardening agents results in three levels of chemical interactions shown in TGA and DTG graphs. Fillers effect on the final characteristics of the composite obtained depends strongly on the level of dispersion obtained. This fact is also dependent on the size and chemical characteristics of them. The predicted interactions do not always occur during the cross-linking process. Also DTG analysis shows the real effect has two possible mechanisms. One of them of weak character and the other that shows a more intense level of interactions between matrix, reinforcement and fillers. Although the existence of these interactions better mechanical behavior cannot always be achieved. When achieved however, Young Modulus increases enormously which is interesting for specific final uses. Flexural behavior is affected by the presence of GO or rGO, but due to the 3D configuration achieved it is difficult to observe a clear influence of the chemical groups in filler particles.
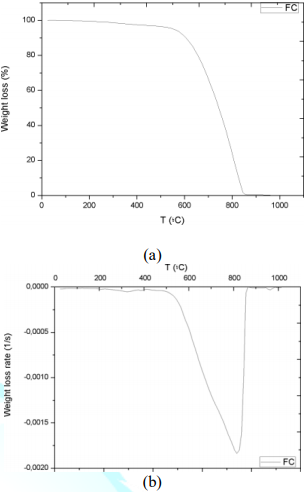
Figure 9: Thermogravimetric analysis of the carbon fiber: (a) TGA curve; (b) DTG plots.
The role of interfaces is clearly shown in SEM pictures. The chemical groups of the GO particles do not show a coupling agent effect on the system. And results show that the interfaces development during the curing process is one of the most important facts in improving mechanical properties. The four levels of chemical interactions that exist in the system formed by GO and rGO when incorporated into a composite material based on carbon fiber and different epoxy resins. Chemical character of systems X/Y/Z-GO/rGO-CF does not strongly affect the final interactions formed in the composite material. The hardening agent has a great influence than the chemical character of rGO and GO. Improving mechanical properties is just a matter of finding particles with functional groups designed specifically to the corresponding resin and/or the substrate used. Carbon fiber has no possibilities of dealing with this approach due to its chemical inertness. The resonance between the aromatic rings is not the most appropriate way to try to improve the interactions in a composite material. The use of larger sized particles faces with the inert interface of the resin because of its very poor chemical interactions. Therefore, just in this specific case, and probably for the first time, chemical composition of the epoxy resins used has been reported. The main idea is to detect with are the active chemical groups on the resin structure and, from the information offered by XPS, try to include GO and rGO nanoparticles into the main structure of the resin, using chemical interactions between chemical groups.
Acknowledgments
The authors would like to show their appreciation to Ruffini S.L. for then financial support, to Zwick S.L. and Isidoro Soto for permission to use their testing machine and publish the results and to Mel Composite S.L. and Torras Suministros Industriales S.L. for providing the material employed in the present study.
References
1. Kong C, Bang J and Sugiyama Y. Structural investigation of composite wind turbine blade considering various load cases and fatigue life (2005) Energy 30:2101-2114.
2. Triantafillou T and Plevris N. Strengthening of rc beams with epoxy-bonded fibre- composite materials (1992) Mater Struct 25:201-211.
3. Botelho EC, Silva RA, Pardini LC and Rezende MC. A review on the development and properties of continuous fiber/epoxy/aluminum hybrid composites for aircraft structures (2006) Mater Res 9:247-256.
4. Toldy A, Szolnoki B and Marosi G. Flame retardancy of fibre-reinforced epoxy resin composites for aerospace applications (2011) Polymer Degradat Stability 96:371-376.
5. Monfared Zanjani JS, Okan BS, Menceloglu YZ and Yildiz M. Nano- engineered design and manufacturing of high-performance epoxy matrix composites with carbon fiber/selectively integrated graphene as multi-scale reinforcements (2016) RSC Adv 6:9495-9506.
6. Argon A and Cohen R. Toughenability of polymers (2003) Polymer 44:6013-6032.
7. Chand S. Review carbon fibers for composites (2000) J Mater Sci 35:1303-1313.
8. Hughes J. The carbon fibre/epoxy interfacea review (1991) Comp Sci Technol 41:13-45.
9. Dai Z, Zhang B, Shi F, Li M, Zhang Z, et al. Determination of the local chemical structure of graphene oxide and reduced graphene oxide (2010) Adv Mater 22:4467-4472.
10. Fitzer E, Geigl KH, Huttner W and Weiss R. Chemical interactions between the carbon fibre surface and epoxy resins (1980) Carbon 18: 389-393.
11. Montes-Moran MA and Young RJ. Raman spectroscopy study of HM carbon fibres: effect of plasma treatment on the interfacial properties of single fibre/epoxy composites (2002) Carbon 40:845-855.
12. Song W, Gu A, Liang G and Yuan L. Effect of the surface roughness on interfacial properties of carbon fibers reinforced epoxy resin composites (2011) Appl Surf Sci 257:4069-4074.
13. Veedu VP, Cao A, Li X, Ma K, Soldano C, et al. Multifunctional composites using reinforced laminae with carbon-nanotube forests (2006) Nat Mater 5:457-462.
14. Tang LC, Wan YJ, Yan D, Pei YB, Zhao L, et al. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites (2013) Carbon 60:16-27.
15. Wan X, Huang Y and Cheng Y. Focusing on Energy and Optoelectronic Applications: A Journey for Graphene and Graphene Oxide at Large Scale (2012) Acc Chem Res 45:598.
16. Singh V, Daeha J, Zhai L and Das S. Graphene based materials: Past, present and future (2011) Prog Mater Sci 56: 1178.
17. Xu JY, Liu J, Li KD, Miao L and Tanemura S. Novel PEPA-functionalized graphene oxide for fire safety enhancement of polypropylene (2015) Sci Technol Adv Mater 16:025006.
18. Edwards RS and Coleman KS. Graphene synthesis: relationship to applications (2013) Nanoscale 5: 38-51.
19. Pathak AK, Borah M, Gupta A, Yokozeki T and Dhakate SR. Improved mechanical properties of carbon fiber/graphene oxide-epoxy hybrid composites (2016) Composites Sci Tech 135:28-38.
20. Abdullah SI and Ansari M. Mechanical properties of graphene oxide (GO)/epoxy composites (2015) HBRC J 11:151-156.
21. Yavari F, Rafiee MA, Rafiee J, Yu ZZ and Koratkar N. Dramatic Increase in Fatigue Life in Hierarchical Graphene Composites (2010) ACS Appl Mater Interfaces 2:2738-2743.
22. Zhang X, Fan X, Yan C, Li H, Zhu Y, et al. Interfacial Microstructure and Properties of Carbon Fiber Composites Modified with Graphene Oxide (2012) ACS Appl Mater Interfaces 4:1543-1552.
23. Hawkins DA and Haque A. Fracture toughness of carbon-graphene/epoxy hybrid Nanocomposites (2014) Procedia Engineering 90:176-181.
24. Su WFA, Chen KC and Tseng SY. Effects of Chemical Structure Changes on Thermal, Mechanical, and Crystalline Properties of Rigid Rod Epoxy Resins (2000) J Appl Polym Sci 78:446-451.
25. Ma S, Liu X, Fan L, Jiang Y, Cao L, et al. Synthesis and properties of a bio-based epoxy resin with high epoxy value and low viscosity (2014) Chem Sus Chem 7:555-562.
26. Bekyarova E, Thostenson ET, Yu A, Itkis ME, Fakhrutdinov D, et al. Functionalized single-walled carbon nanotubes for carbon fiber- epoxy composites (2007) J Phys Chem C 111:17865-17871.
27. Ogasawara T, Moon SY, Inoue Y and Shimamura Y. Mechanical properties of aligned multi-walled carbon nanotube/epoxy composites processed using a hot-melt prepreg method (2011) Comp Sci Technol 71:1826-1833.
28. Chen, L, Chai S, Liu K, Ning N, Gao J, et al. Enhanced epoxy/silica composites mechanical properties by introducing graphene oxide to the interface (2012) ACS Appl Mater Interfaces 4:4398-4404.
*Corresponding author
Manuel J Lis, Chemical Engineering Department, Universitat Politécnica de Catalunya, Barcelona, Spain, E-mail: manuel-jose.lis@upc.edu
Citation
Gomez GS, Lis MJ, Li J, Coldea P, Prada CLD, et al. Go/rGo as reinforcing nanofiller in carbon fiber/epoxy resin composite systems (2019) Nanomaterial Chem Technol 1: 11-18
Keywords
Carbon fiber, Reinforcing nanofiller, Resin composite system


 PDF
PDF