Highlights
- 9% of Receivers and 2% of Decliners experienced a DFS limiting event, while 3% of Receivers and 2% of Decliners died.
- After controlling for stage at diagnosis and type of oncology care received, no difference was found on the Adjusted Hazard ratio of DFS or mortality between Receivers and Decliners.
- Better clinical predictors among Decliners may be related to no rate differences in recurrence and Mortality between Decliners than Receivers.
Introduction
Approximately 3.5 million women have breast cancer in the U.S. and this number goes up each year; in 2019, an estimated 268, 600 women will be newly diagnosed with breast cancer and 41,760 will die from it [1,2]. However, among these women, about 6-13.8% of women with breast cancer willingly decline recommended chemotherapy [3-5], 9% radiotherapy [6] and 14% hormone therapy [7]. Limited evidences suggest that when women did not receive surgery, relative risk of estimated 10-year mortality was 1.58 times higher than among Receivers, while the relative mortality was 1.54 times higher when women did not receive chemotherapy [8].
In another study, the risk for having another breast cancer among woman who did not receive recommended systemic treatments (i.e., chemotherapy, hormone therapy) was 1.9 times, and risk for death was 1.7 times during an average 7.3 years of follow-up [5]. Recently, Johnson et al. found that 5-year survival rate of breast cancer patient was 58.1% among women who did not receive all treatments, whereas it was 86.6% for women who received all treatments; Cox hazard ratio was 5.68 [9]. However, lack of information still existed on the recurrence and mortality of women who did receive surgery but choose not to receive at least one adjuvant therapy (i.e., chemotherapy, radiotherapy, hormone therapy) after surgery. Overall objective of this study was to compare differences in recurrence and mortality between Receivers and Decliners. In this paper, Receivers indicates women who received all doctor recommended medical breast cancer treatments including surgery, chemotherapy, radiotherapy, and hormone therapy. Decliners are those who voluntarily declined all or part of the doctor recommended adjuvant therapy (i.e., chemotherapy, radiotherapy, and hormone therapy) after receiving surgery.
Methods
Design, sample, setting, and procedures
This study used longitudinal and correlational study design. This study reports results from the secondary analysis of the baseline and 5-year follow up data from the Breast Cancer Integrative Oncology Study, in which 427 women were recruited through integrative oncology clinics in greater Seattle area and the Cancer Surveillance System (CSS) registry in Western Washington State. Sample criteria included: 1) 18 years of age or older, 2) biopsy-pathology verified diagnosis of breast cancer or ductal carcinoma in situ, 3) surgery was conducted, 4) their doctor recommended at least one adjuvant treatment among chemotherapy, radiotherapy, and hormone therapy, 5) clear indication of receiving or declining recommended treatment, and 6) clear abstracted records of the following dates: diagnosis, last visit with medical oncologists or recurrence or second primary cancer diagnosis (for analysis of DFS), and death as applicable.
Instruments
Data sources
Data were collected from participants self-reports (household income and comorbidity), oncologists medical chart review (diagnosis date, last visit date with oncologists, and recurrence date), and Cancer Surveillance System (CSS) registry (age and stage at diagnosis, ethnicity, marital status, Estrogen and Progesterone Receptors status, side of breast cancer, receiving/declining recommended treatments, and last contact/death date if indicated) in Western Washington State.
Data analysis
All data were analyzed using SPSS 20 software and R Version 3.2.2 [10]. The comparison of survival and DFS between Receivers and Decliners was analyzed with Cox proportional hazards regression, using the coxph function in R. For the analysis of DFS time was computed as going from date of diagnosis to time of DFS limiting event or death for those who did die but did not recur (n = 2). Those who did not die or recur were censored at the time last known to be alive and not recurred, i.e. the last visit to the oncologist. Analysis of survival was similar, except that time of death was used as the outcome. These analyses controlled for stage of cancer at diagnosis and type of oncology care received. Kaplan-Meier survival and DFS curves were generated using the survfit function in R.
Ethical Considerations
The original study obtained the permission from the Institutional Human Subjects Review Committee of the Bastyr University and Fred Hutchinson Cancer Research Center and each subject provided informed written consent before participation. For the current study, the committees from Bastyr University, Fred Hutchinson Cancer Research Center and the University of Washington approved to conduct secondary data analysis using a de-identified data set.
Sample Characteristics
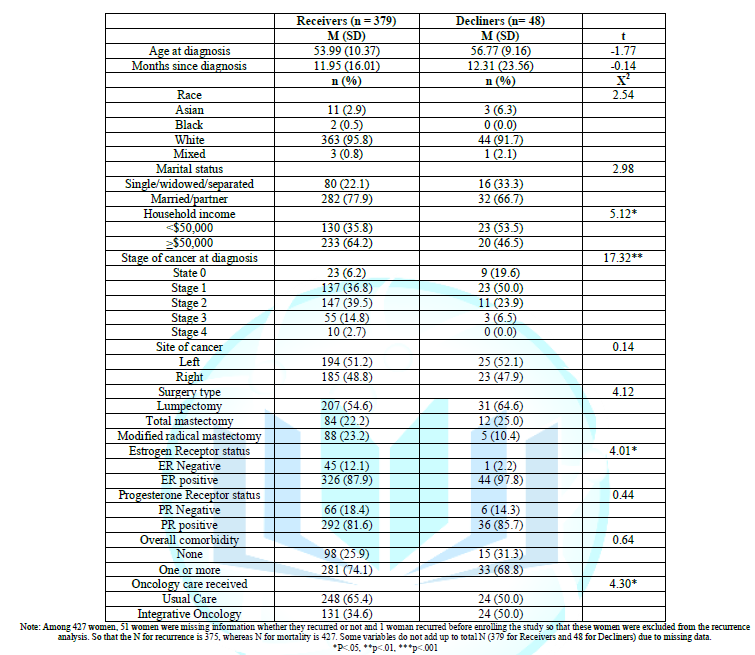
As shown in Table 1, Decliners tended to have less household income, earlier stage of breast cancer at the time of diagnosis, more estrogen positive receptor status, and more frequently received integrative naturopathic oncology care than Receivers. No other differences were found.
Recurrence and Mortality
As depicted in Table 2, overall 31 women (30 Receivers and 1 Decliner) experienced a DFS limiting event, commonly a recurrence of cancer and 13 women (12 Receivers and 1 Decliners) died during 5 years of follow-up. The recurrence rate was 9% (30/334) among Receivers and 2% (1/41) among Decliners, while mortality was 3% (12/379) among Receivers and 2% (1/48) among Decliners. After controlling for stage at diagnosis and type of oncology care received, no difference was found on the Adjusted Hazard ratio of DFS or mortality between Receivers and Decliners. Adjusted Hazard ratio of Decliners relative to Receivers was 0.29 (95% CI; 0.04 – 2.22, p = 0.22) for DFS and 0.50 (95% CI: 0.04, 6.49, p = 0.59) for Mortality.
Figure 1 shows the Kaplan-Meyer curves for comparing disease free survival time between Receivers and Decliners, which was not statistically significant. The x-axis represents time since diagnosis and y-axis represents the fraction of women who are alive and disease free at each time.
All women (100% or 1.0 fraction) start at the top of the y-axis, indicating all women, at the time of diagnosis, have not experienced DFS limiting event or death. The lines go down at the times at which a woman in that group died or experienced a DFS limiting event. Women who did not die or recur are shown in the plot as a small vertical tick mark which indicates the time at which that woman was censored, i.e. the time at which she was last known to be alive and disease-free. Red thin lines represent Decliners and black thin lines represent Receivers.
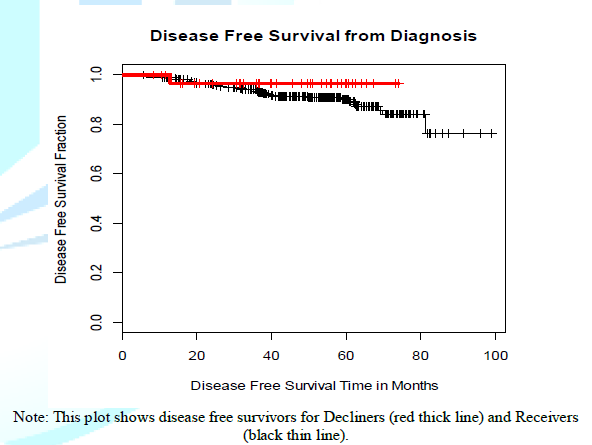
Figure 1: Disease free survival between Receivers and Decliners.
Discussion
Overall, the recurrence rate was 9% among Receivers and 2% among Decliners, while the mortality rate was 3% among Receivers and 2% among Decliners. These rates are much lower than Saquib et al.s finding; they found recurrence rate of 6.63% for Receivers and 19.21% for Decliners and mortality rate of 10.14% for Receivers and 11.30% for Decliners [5]. The Adjusted Hazard Ratio of 0.29 for recurrence and 0.50 for mortality in the current study is much lower than the ratio of 2.35 for recurrence and 3.05 - 5.68 for mortality found in previous studies [5,9]. This difference may be related to data collection time; Saquib et als data were from 1995 - 2000 and Johnson et al.s data were from 2004 - 2013, whereas our data were from 2012 - 2017.
In looking at the difference in findings where we found non-significant differences such that Decliners did not demonstrate poorer outcomes, this may be in part because Decliners in this study tended to have earlier stage of cancer at diagnosis and more estrogen positive receptor status. Suggesting that Decliners in this study may have been aware that their risks of poor outcomes were low when they choose to decline adjuvant treatment. However, for R analyses this study controlled the stage of cancer at diagnosis, and when these adjustments were added the non-significant differences remained but were reduced. It is possible that decliners had more detailed knowledge of their condition from their doctors than we could control for using stage.
Of note also might be the fact that participants in this study were predominantly white women living in an urban area with good access to medical care who generally received guideline consistent care and reported having been very involved in making decisions about their treatment [11]. Our previous study found that overall, 11.2% (48/427) of women declined at least one adjuvant treatment recommended by their medical doctors after their surgery; 8.8% (22/251) chemotherapy, 10.5% (33/314) radiotherapy, and 13.3% (45/339) hormone therapy. Decliners of conventional adjuvant treatment in this study were very involved in their treatment decision making, compared with Receivers. In this context, Decliners in this study were generally women who choose not to receive one or more adjuvant therapies on a therapy by therapy basis and did not include women who were denied treatment based on counter indications due to co-morbidities or poor health, or lack of access [11]. These data may thus present a best-case scenario for self-determination and patient informed decision-making about adjuvant care.
Limitations
Study participants were mostly White Americans and the sample size for Decliners was much smaller than Receivers. Repeating the study with more women who have more diverse, income, educational, ethnic and cultural backgrounds would be important. There were 51 women whose recurrence status was missing in medical record, which decreased sample size. Recording recurrence of all individuals with cancer would be important. And it would be nice if these data are collected by CSS registry. Some Receivers may not 100% adherent to all adjuvant treatments; conversely some Decliners might have received some adjuvant treatments later; however, this information was not collected.
Conclusion
Our study found no significant difference, suggesting that in some cases Decliners may be patients with good prognosis; receiving treatment in their case would represent over-treatment. To our knowledge, this is the first study that demonstrated no statistically significant difference in the rate of recurrence and death between Receivers and Decliners. This finding is not congruent with earlier studies that have shown that Decliners were at a higher risk of recurrence or death. And it is not clear why the results were not consistent, although we have attempted to present some possible explanations.
Acknowledgement
This paper discusses secondary analysis using the data from the Breast Cancer Integrative Oncology: Prospective Matched Controlled Outcomes Study which was awarded to Drs. Andersen and Standish, from NIH, NCCAM, R01 AT005873.
References
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, et al. Cancer treatment and survivorship statistics, 2014 (2014) CA Cancer J Clin 64: 252-271.http://dx.doi.org/10.3322/caac.21235
- https://seer.cancer.gov/statfacts/html/breast.html
- Greenlee H, Neugut AI, Falci L, Hillyer GC, Buono D, et al. Association between complementary and alternative medicine use and breast cancer chemotherapy initiation: The Breast Cancer Quality of Care (BQUAL) Study (2016) JAMA Oncol 2: 1170-1176. http://dx.doi.org/10.1001/jamaoncol.2016.0685
- Neugut AI, Hillyer GC, Kushi LH, Lamerato L, Leoce N, et al. Non-initiation of adjuvant chemotherapy in women with localized breast cancer: the breast cancer quality of care study (2012) J Clin Oncol 30: 3800-3809. http://dx.doi.org/10.1200/jco.2012.43.8168
- Saquib J, Parker BA, Natarajan L, Madlensky L, Saquib N, et al. Prognosis following the use of complementary and alternative medicine in women diagnosed with breast cancer (2012) Complement Ther Med 20: 283-290. http://dx.doi.org/10.1016/j.ctim.2012.04.002
- de Csepel J, Tartter PI, and Gajdos C. When not to give radiation therapy after breast conservation surgery for breast cancer (2000) J Surg Oncol 74: 273-277. https://doi.org/10.1002/1096-9098(200008)74:4<273::AID-JSO6>3.0.CO;2-V
- Brett J, Fenlon D, Boulton M, Hulbert-Williams NJ, Walter, FM, et al. Factors associated with intentional and unintentional non-adherence to adjuvant endocrine therapy following breast cancer (2018) Eur J Cancer Care (Engl) 27. http://dx.doi.org/10.1111/ecc.12601
- Chang EY, Glissmeyer M, Tonnes S, Hudson T and Johnson N. Outcomes of breast cancer in patients who use alternative therapies as primary treatment (2006) Am J Surg 192: 471-473. http://dx.doi.org/10.1016/j.amjsurg.2006.05.013
- Johnson SB, Park HS, Gross CP and Yu JB. Use of alternative medicine for cancer and its impact on survival (2018) J Natl Cancer Inst 110. http://dx.doi.org/10.1093/jnci/djx145
- http://www-01.ibm.com/software/analytics/spss/
- Kim E, Andersen MR, and Standish LJ. Receiving/declining adjuvant breast cancer treatments and involvement in treatment decision-making (2019) Complement Ther Med 43: 85-91. http://dx.doi.org/10.1016/j.ctim.2019.01.012
*Corresponding author: Eunjung Kim, Department of Family and Child Nursing, University of Washington, USA, Tel: 206-543-8246, Fax: 206-543-6656, Email: eunjungk@uw.edu
Citation: Kim E, Standish LJ and Andersen MR. Recurrence and mortality rates among receivers and decliners of conventional adjuvant breast cancer treatments (2019) Edelweiss Cancer OA 1: 33-36Keywords
Breast cancer, Recommended conventional treatments, Receivers, Decliners, Recurrence, Mortality


 PDF
PDF
