Introduction
Tuberculosis (TB) is an infection caused by mycobacterium tuberculosis; commonly it affects the lungs (pulmonary), but can affect any organ in the body [1]. World Health Organization (WHO) considered that, tuberculosis (TB) is a global public health problem affected 10 million people worldwide, with 1.6 million died. More than, 95% of the cases and 97% of deaths occur in development countries [1,2]. The disease continues to be a problem in industrialized countries as well, mostly in immigrants populations, in elderly individuals whose latent infection is reactivated.
TB is spread from person to person through the air. When people with lung TB cough, sneeze or spit, they propel the TB germs into the air. A person needs to inhale only a few of these germs to become infected. Several studies reported that, transmission of TB infection found to be High among students and healthcare workers as a nosocomial transmission, when biosafety guidelines were not strictly followed [3,4]. It was estimated that, about one-quarter of the worlds population has latent TB, which means people have been infected by TB bacteria but are not yet ill with the disease. The risk of transmission of Mycobacterium tuberculosis from patients to Health Care Workers (HCWs) is greatest in facilities with a high burden of infectious Tuberculosis (TB) cases [5]. Transmission of TB in health care facilities can be reduced or prevented with the implementation of effective infection control measures [6].
There are other risk groups whom are susceptible to TB infection, such as intravenous drug users, patients with end stage renal disease, HIV positive patients, the homeless. Several studies have demonstrated the importance of M. tuberculosis transmission in students and among nurses [4-7] Nosocomial transmission, especially between TB/HIV co-infected patients and health care workers, when biosafety guidelines were not strictly followed [3,4]. Despite the importance of LTBI, the tuberculin skin test was, until recently, the only test available for its diagnosis [8]. The TST measures hypersensitivity response to Purified Protein Derivative (PPD), a crude mixture of antigens, many of which are shared among M. Tuberculosis, Mycobacterium bovis Bacilli Calmette-Guérin (BCG) and several Non Tuberculous Mycobacteria (NTM). The TST has limitations with respect to accuracy and reliability [8,9]. Advances in genomics and immunology have led to a promising alternative, the in vitro interferon γ(IFN- γ) assay [8,9], based on the concept that T cells of infected individuals release IFN- γ.
The QuantiFERON-TB assay (Cellestis Limited, Carnegie, Australia) was the first commercially available IFN- γ assay. The PPD-based QuantiFERON-TB test was approved by the US Food and Drug Administration in 2001 [10-12] demonstrated higher prevalence of tuberculin skin testing among health care workers than general population [13-15], necessitate effective infection control measures [6].
Currently, the CDC guidelines recommend that HCWs receive tuberculin testing on entry to the workplace and an assessment should be made to assign a risk category to HCWs in an institution and thus the frequency of screening should be determined according to the risk [16]. Each institution should use its own surveillance data from tuberculin skin testing to assess the specific risk category of its HCWs. Many healthcare institutions have mandatory annual tuberculin screening for their HCWs. Annual screening provides significant protection for HCWs as most active cases develop within the first few years after the initial infection, however CDC did not make specific recommendations concerning treatment of the latent infection [17].
Health care workers screening in Saudi Arabia revealed that, the overall prevalence of positive TST among HCWs at king Abdulaziz university hospital was 78.9%, 60.0% for Saudi Arabian compared with 81.0% for non-Saudi Arabians [18]. In Yemen, the prevalence of tuberculosis among adults population (15-45 years) was 136/100,000 with mortality rate about 21/100,000 [2,19]. Unfortunately, prevalence rates of TB among health care workers in Yemen are not known, even though TB is considered as one of the most common chronic infectious diseases in the country [19].
This study was carried out to identify the prevalence of latent tuberculosis infection among health care workers in the main referral hospital (TMGH) at Sanaa City Yemen. Using tuberculin skin test rather than QuantiFERON- test for two reasons firstly there is an agreement between both tests in various reports [20-22] and secondly, the cost of QuantiFERON- test is higher, in the presence of the lack of resources, and also the absence of the political commitment in the health authority of this country.
Objective
Explanation for including both general and specific objectives:
These are fundamental in any health research. General objective is a broad statement of what the researcher hopes to accomplish. They create a setting for the research. Specific objectives are statements of the research question(s). After statement of the primary objective, secondary objectives may be mentioned.
General objective
To determine the prevalence of LTB among health care workers in Al- Thawra Modern General Hospital, Sanaa -Yemen.
Specific objectives
- To determine the occupational risk of mycobacterium TB infection among health care workers.
- To identify higher risk group for acquiring TB among HCWs.
- To suggest control measures to prevent transmission of TB to HCWS
Methods
This study was a descriptive - cross sectional study carried out in Al Thawra Modern General Hospital; which is a referral hospital located in the center of capital of Yemen. The hospital well equipped and has 1000 beds.
All HCWs working at the medical, surgical, emergency, intensive care units, laboratory and radiology departments were included in this study. Any participant with a history of old TB or positive TST before joining the hospital, any family history of TB or close contact more than one year, any HCWs with employment or training less than one year in the hospital or refused to participation in the study were excluded.
A questionnaire was design to document demographic data such as age sex, profession, department, duration of work in the hospital, past history and family history of tuberculosis. Pilot test of questioner was done and ambiguous question were reworded.
We excluded health care worker if his or her age below 18 years, working in the hospital less than 1 year, has history of tuberculosis or resent contact to Tuberculous patients and those refusing the TST or refused to repeat the second step test.
TST was done by a single investigator using the standard Monteux intradermal injection. The standard tuberculin test consists of 0.1 ml (5 tuberculin units) of PPD administered intracutaneously, usually in the forearm, using a 26 gauge needle after sterilization with normal saline. The reaction is read 48 to 72 hours after injection and the widest axis of indurations was measured by a standardized palpation method with a flexible ruler after a period of 48-72 hours, by the same investigator. The conduct and interpretation of tuberculin skin tests were based on current guidelines of the CDC Committee on Latent Tuberculosis Infection [23].
For the purpose of our analysis, two cut–off point used to define the positive TST reaction, an indurations of 10 mm or greater regarded as positive and an indurations of 15mm or more is regarded as strong positive. Those with negative result advised to come after 1-2 weeks for second step TST & those who agreed, underwent 2nd step TST after 1-2 weeks. Those reacted strong positive TST were investigated to rule out active TB [23,24].
BCG vaccination history was ignored in this study, because the health care workers are high risk group and booster doses of BCG is not performed in Yemen routinely. Data collected from all participants were coded and entered in the computer and analyzed using SPSS software. Frequency tables and determination of significant differences among variables were performed by the chi-squared test and Student t-test, P value ≤ 5 considered significance.
Ethical Consideration
- All participants informed in details regarding t the nature of the study, the risks and expected discomfort of TST.
- The participants information will be secured.
- This study was approved by Hospital research committee
- Written informed consent obtained from each participant.
Results
The total number of health workers in the hospital were 466, Eligible cases whom fulfilled the including criteria were 426. The remaining either excluded or not present at the time of study. Of them 232 (54.5%) were males and 194 (45.5%), were females with a ratio of 1.2:1. Of the 426 participants 215 (50.5%) were positive from the 1st step TST and 211 (49.5%) were 1st step TST negative and needed to do the 2nd step. However, 40 (19%) were unable to repeat the TST for different reasons. Thus, 171 (81%) were able to do the 2nd step TST, of whom 54 (32%) turned to be positive and 117 (68%) had negative result. The total number found to be positive from first and second steps were 269 (70%) and 117 HCWs (30%) were negative. Out of the 269 positive cases, 144 (37%) were strong positive (>15mm), 119 (31%) were just positive (10mm-15mm), 6 (2%) were strong positive with skin ulceration. those with strong positive TST were screened for active TB and only 2 were found to have radiological evidence of apical fibrosis, one of them found to has active TB (Figure 1).
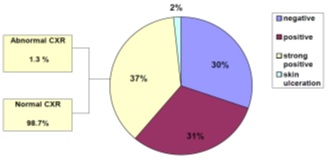
Figure 1: Tuberculin skin test reaction results.
Distribution of positive results among age group of both sexes is shown in Figure 2. The prevalence of positive TST among young age group (18-28), were 56% while the elder group (40-50 years) were 89% and this rate reach 100% among GHCWs of above 50 years old.The positive TST were 197 (70%) Yemeni HCWs, 62 (66%) Indian, 6 (100%) Philippines and 4 (100%) other nationality, there is no significant P value 0.362 (Table 1).
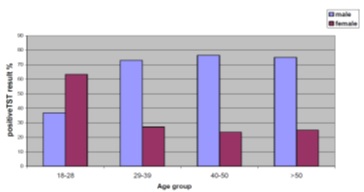
Figure
2: Distribution of positive TST according to the age
& sex.

Table 1: Distribution of positive TST according to the sex & nationality.
The distribution of positive TST among HCWs in the different departments is shown in Table 2. There were no significant differences between them, with prevalence rate in the surgical department, laboratory, radiology medical wards, emergency department accounted for (78%,77%,74%,71%,66%) respectively. However, the lower rate 48% was found among HCWs in the emergency intensive care unit. Regarding to employment (professional) category, the highest positive result was recorded among radiology assistants (91%) followed by laboratory workers (80%), doctors (76%), nurses (65%), radiologists (59%), administrators (58%) and respiratory therapist (58%), the differences found to be significance with P. value of 0.0184 (Table 3).
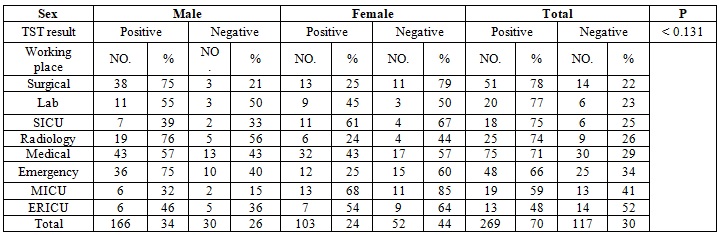
Table 2: Distribution of positive TST according to the sex & working place.
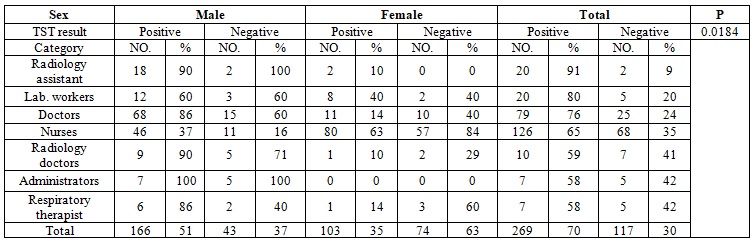
Table 3: Distribution of positive TST according to the sex and employment category.
There is a deference in TST result in female with the use of female veil (the dress used by the female in the Islamic countries in which they cover their body with it including their face leaving only their eyes) which was 52% compared to females did not use veil (55%), but this differences did not have statically significant with P value <0.80.
Discussion
In this study the prevalence of LTBI among HCWs at Al-Thawra Modern General Hospital Sanaa Yemen was 70%. This high rate is expected in the country where prevalence of TB among general population reached to 136/100.000 and incidence of TB is about 82/100,000 [2,19].
This make HCWs in the hospital expose to high incidence of TB infection. Reports from Turkey showed that the incidence of TB was 199.9/100,000 among health care workers, compared with 40.8/100,000 in the general population, giving a relative risk of approximately 5 times greater among health care workers than in the general population [25]. Similar result was reported from Poland where high rate of TB incidence among HCWs was 204-721/100,000, compared to general population which was 24.3/100,000 [26]. In Malawi, it was found that the incidence of TB among health care workers in 40 hospitals that treat TB patients was twelve times greater than that of the general adult population [27]. In a study conducted in Brazil, it was found that nurse students were more susceptible to infection with M. tuberculosis than the general population at a rate of 10.5% and 0.5% respectively [28]. While in Finland, the risk is lower among health care workers than in the general population because Finland has an excellent TB control program. However, the risk of developing TB among HCWs is dependent on several factors such as occupational category, age and use of TB infection control measures in the workplace [29,30].
In our study the positive result of STT was higher in males (62%) than the female (38%), this could be explained by the fact that the female in our country joined the health profession too late compared to their male counterpart another peculiar culture in Yemeni females, that most of them wearing the traditional veil (alhijab or alnikab) which may possibly protect them against infection.
Moreover, the frequency of TST was higher in female expatriate nurses, and Yemeni female nurses not wearing the veil (55%) than in those with veil (52%) with no veil has a possible protective effect on female nurses against TB infection, statistically significant difference.
These findings may suggest that the veil has a possible protective effect on female nurses against TB infection, but this point needs further study. In this study we found high prevalence of positive TST among elder age group ≥ 40years and in those with longer work duration which is consistent with the findings reported in HCWs in high incidence countries like (India, Russia, and Georgia) [31-33]. There were variations of LTBI among different employment categories, in our study, with radiology technicians was caught the highest rate of LTBI (91%) and the lowest rate was among administrators.
Similar results had been reported from other places [34,35]. These variations could by related to environmental factor and or close contact with TB patients. The environment in Radiological rooms are often poorly ventilated, therefore, droplet nuclei may stay in the air for hours or even days. While the lowest rate among administrators is due to less contact with TB patents and has good ventilation. The lowest rate of LTBI among administrators is due to less contact with patients. The respiratory therapist has lower rate of positive TST in our study unlike the other reports as in Poland this is because this occupation is new in our hospital (TMGH) which started 2 years ago and more time is needed to have positive conversion. Regarding types of professionals we found surgeons and laboratory personnel acquired the high rate of LTBI (78% & 77%) respectively. These high rates could be related to nature of work in our hospital as it is general referral hospital, most patients with complicated TB admitted to surgical word for surgical intervention such as, chest empyema and lung cavity. The procedures and manipulation of lesion/s produce minutes droplets which are more penetrable than larger droplets and expose the surgeons to LTBI [2]. On the other hand Laboratory personnel received body fluid like blood, sputum, fluid from pleural abdomen, pus etc., from different department for bacteriological analysis and exposed them to acquire TB infection, especially in hospital with lack of infection control program. Similar result has been reported from other study [35].
In our study the doctors have higher percentage of TST than the nurses in contrast to other studies, where nurses have been found to have a higher risk for developing TB [36,37] in spite of the fact that nurses are the first point of contact with patients in the out-patient departments and are continuously in contact with patients in hospital wards. They also take sputum samples, including those obtained by induction from infectious patients, increasing their risk of exposure.
This paradox could be explained by the fact that majority of the nurses are females and once they got married they quit the job so they dont stay longer in the hospital in addition to the fact that most of the females are wearing the veil which could work as a mask for them. High suspicion of tuberculosis by the clinician, adequate infection-control measures by the hospital authorities and early identification of latent tuberculosis infection in healthcare workers by occupational specialists form the essential components of a comprehensive package to prevent tuberculosis in healthcare workers [17].
Conclusion
We found high LTBI rates among HCWs at Al-Thawra Modern General Hospital in Sanaa Yemen with male predominance with higher positivity among high risk working places & risky occupation. This suggests that some TBI develops via in-hospital infection.
In order to enhance the protection of both health care workers and hospitalized patients, effective control and preventive measures for TB, including yearly tuberculin testing of health care workers, should be considered.
Acknowledgement
We are thankful to all the health workers at Althawra Teaching Hospital for participation in this study. Our thanks to Al Awlaki lab for their great help in supplying the tuberculin tests to all the participants.
References
1. Centers for Disease Control and Prevention. Estimates of future global tuberculosis morbidity and mortality (1993) MMWR Morb Mortal Wkly Rep 42: 961-964.
2. Dye C, Scheele S, Dolin P, Pathan V and Raviglione MC. Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence and mortality by country (1999) JAMA 282: 677-686.
3. Sokolove PE, Mackey D, Wiles J and Lewis RJ. Exposure of emergency department personnel to tuberculosis: PPD testing during an epidemic in the community (1994) Ann Emerg Med 24: 418-421. https://doi.org/10.1016/S0196-0644(94)70178-4
4. Zaza S, Blumberg HM, Beck-Sagué C, Haas WH, Woodley CL, et al. Nosocomial transmission of Mycobacterium tuberculosis: Role of health care workers in outbreak propagation (1995) J Infect Dis 172: 1542-1549.
5. Harries AD, Maher D and Nunn P. Practical and affordable measures for the protection of health care workers from tuberculosis in low-income countries (1997) Bull World Health Organ 75: 477-489.
6. Blumberg HM, Watkins DL, Berschling JD, Antle A, Moore P, et al. Preventing the nosocomial transmission of tuberculosis (1995) Ann Intern Med 122: 658-663.
7. Maciel EL, Meireles W, Silva AP, Fiorotti K and Dietze R. Nosocomial Mycobacterium tuberculosis transmission among healthcare students in a high incidence region, in Vitória, State of Espírito Santo (2007) Rev Soc Bras Med Trop 40: 397-399. http://dx.doi.org/10.1590/S0037-86822007000400004
8. Huebner RE, Schein MF and Bass JB Jr. The tuberculin skin test (1993) Clin Infect Dis. 17: 968-975.
9. Andersen P, Munk ME, Pollock JM and Doherty TM. Specific immune-based diagnosis of tuberculosis (2000) Lancet 356: 1099-1104. https://doi.org/10.1016/S0140-6736(00)02742-2
10. Mazurek GH and Villarino ME. Guidelines for using the QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. Centers for Disease Control and Prevention (2003) MMWR Recomm Rep 52: 15-18.
11. Chan ED, Heifets L and Iseman MD. Immunologic diagnosis of tuberculosis: a review (2000) Tuberc Lung Dis 80: 131-140. https://doi.org/10.1054/tuld.2000.0243
12. Jackett PS, Bothamley GH, Batra HV, Mistry A, Young DB et al. Specificity of antibodies to immune dominant mycobacterial antigens in pulmonary tuberculosis (1988) J Clin Microbiol 26: 2313-8.
13. Maciel EL, Viana MC, Zeitoune RC, Ferreira I, Fregona G, et al. Prevalence and incidence of Mycobacterium tuberculosis infection in nursing students in Vitória, Espírito Santo (2005) Rev Soc Bras Med Trop. 38: 469-472. https://doi.org/S0037-86822005000600004
14. Harries AD, Nyirenda TE, Banerjee A, Boeree MJ and Salaniponi FM. Tuberculosis in health care workers in Malawi (1999) Trans R Soc Trop Med Hyg 93: 32-35. https://doi.org/10.1016/S0035-9203(99)90170-0
15. Hosoglu S, Tanrikulu AC, Dagli C and Akalin S. Tuberculosis among health care workers in a short working period (2005) Am J Infect Control 33: 23-26. https://doi.org/10.1016/j.ajic.2004.07.013
16. Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities,1994 (1994) MMWR 43: 1-132.
17. Vries G, ebek MMGG and Weezenbeek L. Healthcare workers with tuberculosis infected during work (2006) Eur Respir J 28: 1216-1221. https://doi.org/10.1183/09031936.06.00039906
18. Koshak EA and Tawfeeq RZ. The prevalence of positive tuberculin skin reactions at King Abdulaziz University Hospital, Saudi Arabia (2003) East Mediterr Health J 9: 1034-1041.
19. https://www.who.int/tb/publications/global_report/tb18_ExecSum_web_4Oct18.pdf
20. Mazurek GH and Villarino ME. Guidelines for using the QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection (2003) Centers for Disease Control and Prevention. MMWR Recomm Rep. 52: 15-18.
21. Chan ED, Heifets L and Iseman MD. Immunologic diagnosis of tuberculosis: A review (2000) Tuberc Lung Dis 80: 131-140. https://doi.org/10.1054/tuld.2000.0243
22. Jackett PS, Bothamley GH, Batra HV, Mistry A, Young DB et al. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis (1988) J Clin Microbiol 26: 2313-2318.
23. https://www.cdc.gov/mmwr/preview/mmwrhtml/00038873.htm
24. Jerant A, Bannon M and Ritteenhouse S. Identification and management of tuberculosis (2000) American family physician 61: 2667–2678. https://doi.org/10.1016/j.ajic.2004.07.013
25. Hosoglu S, Tanrikulu AC, Dagli C and Akalin S. Tuberculosis among health care workers in a short working period (2005) Am J Infect Control 33: 23-26. https://doi.org/10.1016/j.ajic.2004.07.013
26. Demkow U, Broniarek-Samson B, Filewska M, Lewandowska K, Maciejewski J, et al. Prevalence of latent tuberculosis infection in health care workers in Poland assessed by interferon-Gamma whole blood and tuberculin skin test (2008) J Physiol Pharmacol 6: 209-217.
27. Harries AD, Nyirenda TE, Banerjee A, Boeree MJ and Salaniponi FM. Tuberculosis in health care workers in Malawi (1999) Trans R Soc Trop Med Hyg 93: 32-35. https://doi.org/10.1016/S0035-9203(99)90170-0
28. Maciel EL, Viana MC, Zeitoune RC, Ferreira I, Fregona G, et al. Prevalence and incidence of mycobacterium tuberculosis infection in nursing students in Vitória, Espírito Santo (2005) Rev Soc Bras Med Trop 38: 469-472. https://doi.org/S0037-86822005000600004
29. Alonso-Echanove J, Granich RM, Laszlo A, Chu G, Borja N, et al. Occupational transmission of mycobacterium tuberculosis to health care workers in a university hospital in Lima, Peru (2001) Clin Infect Dis 33: 589-596. https://doi.org/10.1086/321892
30. Barret-Connor E. The epidemiology of tuberculosis in physicians (1979) JAMA 241: 33-38.
31. Pai M, Gokhale K, Joshi R Dogra S, Kalantri S, et al. Mycobacterium tuberculosis infection in health care workers in rural India: Comparison of a whole-blood interferon gamma assay with tuberculin skin testing (2005) JAMA 293: 2746-2755. https://doi.org/10.1001/jama.293.22.2746
32. Mori T, Sakatani M, Yamagishi F Takashima T, Kawabe Y, et al. Specific detection of tuberculosis infection: An interferongamma-based assay using new antigens (2004) Am J Respir Crit Care Med 170: 59-64. https://doi.org/10.1371/journal.pmed.0030494
33. Joshi R, Reingold A L, Menzies D and Pai M. Tuberculosis among health care workers in low- and middle-income countries: a systematic review (2006) PLoS Med 3: 494. https://doi.org/10.1371/journal.pmed.0030494
34. Naidoo S and Jinabhai CC. TB in health care workers in KwaZulu-Natal, South Africa (2006) Int J Tuberc Lung Dis. 10: 676-682.
35. Arcia-Garcia ML, Jiminez-Corona A, Jiminez-Corona ME, Ferreyra-Reyes L, Martínez K, et al. Factors associated with tuberculin reactivity in two general hospitals in Mexico (2001) Infect Control Hosp Epidemiol 22: 88-89. https://doi.org/10.1086/501869
36. Hosoglu S, Tanrikulu AC, Dagli C, Akalin S. Tuberculosis among health care workers in a short working period (2005) Am J Infect Control 33: 23-26. https://doi.org/10.1016/j.ajic.2004.07.013
37. Jiamjarasrangsi W, Hirunsuthikul N and Kamolratanakul P. Tuberculosis among health care workers at King Chulalongkorn Memorial Hospital, 1988-2002 (2005) Int J Tuberc Lung Dis 9: 1253-1258.
*Corresponding author
Faiza A, Associate professor of internal Medicine, Sanaa University, Yemen, E-mail: faiza.askar@yahoo.com
Citation
Faiza A, Khaled A, Mohammed B and Abdalhafed A. Prevalence of Latent Tuberculosis Infection (LTBI) among health care workers at Al-Thawrah modern general hospital, Sanaa-Yemen 2016 (2019) Nursing and Health Care 4: 1-5


 PDF
PDF