Introduction
Physicians during their role of patient pain management frequently prescribe opioids. Clinicians have an ethical obligation to prescribe responsibly yet cautiously to diminish the potential for opioid diversion and help minimize the growth of the current epidemic of opioid abuse. Analgesic opioid therapy has been the cornerstone of pharmacotherapeutic management of acute and chronic pain. Ideally, opioid analgesics are prescribed by balancing beneficial and adverse effects. Further, the foot and ankle specialist must remember that comfort is the ultimate goal when using any medication, including opioids, to manage pain [1,2]. Opioid selections are based on patient specific factors such as age, renal function, and sex differences.
The use of an opioid agent requires a practitioner to be comfortable with their use, especially in the presence of both either demographic or disease states like age, obesity, diabetes mellitus, kidney disease, congestive heart disease, and sex differences. Clinicians have been directed to select immediate-release opioid formulations over extended-release or long-acting opioids because they are safer, regardless of whether the drug is used for acute or long-term treatment.
Due to a medication’s pharmacodynamics and pharmacokinetic profile or a patient’s response to an opioid agent, no single opioid analgesic product may be a perfect choice for a clinician to rely on, in order to treat all types of pain. Although no opioid seems to be superior in relieving pain, certain products are clearly inferior because of increased risks of toxic effects [1-3]. Opioid pharmacologic regimens must be individualized based on subjective, objective, and clinical findings. As part of opioid stewardship, this review highlights and describes opioid pharmacodynamic and pharmacokinetic especially in the presence of disease states like diabetes mellitus, kidney disease, congestive heart disease, and sex differences to assist and empower the podiatric physician to prescribe an opioid agent for maximizing opioid analgesic effects and decrease opioid possible adverse effects.
First of all, alterations in pharmacokinetics and pharmacodynamics due to age, illnesses, and sex will be presented as a foundation. Second, building on these clinical alterations in pharmacokinetics and pharmacodynamics that will be applied, and opioid pharmacology will be presented. Finally, methods of clinical coping centered on opioid prescribing will be applied to patients who may present with alterations in pharmacokinetics secondary to age, illness, or sex.
Pharmacokinetic Changes
Given, the change in physiological, pharmacological, and psychological aspects of the aging adult of the symptom of pain in the elderly population is especially difficult to treat. Opiates and opioids are the mainstay of pain treatment throughout all age groups, but special attention must be paid to the efficacy and side effects of these powerful drugs when prescribing to a population with impaired metabolism, excretion, and physical reserve [4]. As a patient becomes elderly, important age-related changes might alter opioid drug pharmacokinetics and result in unwanted side effects.
The process aging is characterized by structural and functional changes affecting all organ systems, which results in reduced homeostatic capacity over time. Although the function of a particular system may be maintained during resting conditions, the reduction of functional reserve is responsible for an increased vulnerability to stress [5]. Changes in body composition and that in hepatic and renal function are responsible for an increase in the volume of distribution of lipid-soluble drugs and reduced clearance of lipid-soluble and water-soluble drugs. All these changes lead to a prolong plasma elimination half-life, leading to greater drug plasma levels. Moreover, significant pharmacodynamics changes occur that cause an increased sensitivity to many drugs. Finally, a reduced body functional reserve itself also leads to an increase in sensitivity by impairing homeostatic compensatory mechanisms [5].
Opioids are highly varied and generally thought to possess similar pharmacokinetic activity. Opioids are rapidly absorbed in the gut, have high rate of first pass in the liver, are conjugated in the liver, have metabolites, and vary in distribution based on their differing protein affinity, and then they are excreted via bile to feces or via kidneys. Opioid pharmacodynamics effects are complex and depend upon poorly measured variables such as receptor function and intracellular response, which can alter drug action. Pharmacokinetic actions of drug absorption, distribution, and elimination are more measurable [5,6].
As a patient ages, the rate at which certain drugs are absorbed can be altered in adults because of a decrease in gastrointestinal transit time and increase gastric pH secondary to the use of proton pump inhibitors, H2 receptor antagonists, or antacids to treat age related gastrointestinal aliments. With aging, there are changes in body composition, such as increase in adipose tissue, decrease in lean body mass, and decrease in total body water. These changes can affect drug distribution. Therefore, lipophilic drugs tend to have greater volume of distribution and can take more time to be eliminated from the body. Aging can also bring reduction in hepatic blood flow and volume, which can decrease metabolism of drugs [5-8].
Additional impairments in drug metabolism can occur with impaired Phase I reactions, which include oxidation, hydroxylation, and dealkylation. This can specifically reduce the first pass effect of opiates in the elderly. Elimination of drugs can be altered with age-related reductions in renal blood flow and Glomerular Filtration Rate (GFR). For opiates that have primary renal clearance, such as morphine and hydromorphone, decreases in GFR lead to more side effects [5-9].
Obesity which has been identified to be a clinical trait of Type 2 diabetes mellitus affects all four aspects of pharmacokinetics. For example, when a clinician utilizes weight-based dosing they must accept that a drug’s fate is based on total body weight that may be affected by obesity resulting in under dosing or overdosing patients, depending on the characteristics of the drug. The pharmacodynamics profile of drugs may also be affected, e.g. the risk of respiratory depression and loss of airway patency is greater with sedatives and narcotics. Careful therapeutic drug monitoring is important in obese patients. Morbidly obese people are often excluded from clinical trials during the drug development process, so data is limited on the correct dosing of many drugs.
Therefore using clinical judgment, combined with interpretation of drug pharmacokinetics is often required by the clinician when they prescribe a medication. Obesity causes increased absorption of oral medications, increased gastric emptying and a decreased subcutaneous absorption is due to poor subcutaneous blood supply. Decreased subcutaneous absorption is due to poor subcutaneous blood supply. Intramuscular administration may fail if needles are too short to pierce the skin. Opioid distribution is markedly affected by ratio of adipose tissue to lean body mass if a drug has high lipid solubility then its volume and distribution will result in an accumulation in fat stores [10]. Literature outlining the research between medication fates within the disease process of Diabetes Mellitus is cited below. Diabetes mellitus has been found to affect protein, lipid, and carbohydrate metabolism, and the biochemical pathways that are involved in drug biotransformation. Four principles of pharmacokinetics that may be influenced by diabetes mellitus include absorption, distribution, biotransformation, and excretion. Diabetic changes in subcutaneous and muscle blood flow, and delayed gastric emptying may influence the way a drug is absorbed [11-16].
Non-enzymatic glycation of albumin secondary to diabetes mellitus may affect a medication’s distribution within the body. Gastric emptying is frequently abnormal in patients with long-standing type 1 and type 2 diabetes mellitus. Symptoms commonly associated with delayed gastric emptying include nausea, vomiting, bloating, and epigastric pain. These patients are also at risk of malnutrition, weight loss, impaired drug absorption, disordered glycemic control, and having a poor quality of life. It although many studies have reported diabetes-mediated changes in gastric emptying time, the magnitude of the delay is modest, and at this time, some authors may not consider it clinically important [11-13].
Drug metabolism is enzyme-mediated structural modification to a drug that changes its biological activity and/or water solubility. These enzymatic reactions result in metabolites that may be active or rendered inactive. The gastrointestinal wall, lungs, liver, and blood possess enzymes that metabolize drugs. Drug metabolism by the liver occurs through one or both biotransformation reactions classified as either Phase I or Phase II reactions. Building on the assertion centered on the direct relationship between diabetes mellitus and obesity, the effect of obesity on cytochrome P450 appears to be isozyme-specific with the activity of cytochrome P450 3A4 decreasing. The clearance of Cytochrome P450 (CYP) 3A4 substrates is lower in obese patients in comparison with non-obese patients [14-16].
Conversely, researchers saw trends indicating higher clearance values via the following Cytochrome P450 isoenzymes: CYP1A2, CYP2C9, CYP2C19 and CYP2D6.16 Opioid drug-drug interactions are presented to assists clinicians to prevent drug harm and drug misadventure in obese and diabetic patients in Table 1.
There are complex interactions within the body’s neuro-endocrine systems in response to opioids include alterations in the autonomic nervous system (sympathetic and parasympathetic nervous system), the renin-angiotensin-aldosterone system and anti-diuretic hormone. In addition, other mechanisms are also involved, including dehydration, rhabdomyolysis, and urinary retention. Changes in the sympathetic and parasympathetic nervous systems affect the kidney by altering renal blood flow and glomerular filtration rate.
These changes occur at several levels including the heart and kidney. Usually the autonomic nervous system controls vital body functions with the sympathetic and parasympathetic innervation acting antagonistically based on need. In the cardiovascular system, the Sympathetic Nervous System (SNS) increases heart rate and myocardial contractility as well as raises peripheral vascular resistance and arterial blood pressure via vasoconstriction [17].
In chronic kidney diseases, there is an increase in morphine in the mean peak concentration and the area under the concentration-time curve for both active and principle metabolites. With chronic kidney diseases, the metabolites of merperidine are present for longer, can decrease the seizure threshold, and should be avoided for chronic use. Extended effects of codeine and dihydrocodeine with chronic kidney disease have been reported. Specific drug pharmacokinetics and pharmacodynamics may differ between men and women. [18] Soldin and Mattison report that reviews of the Food and Drug Administration’s Adverse Events Reporting System (AERS) suggest that women experience more drug-related adverse events, and often these adverse events are described as more serious.
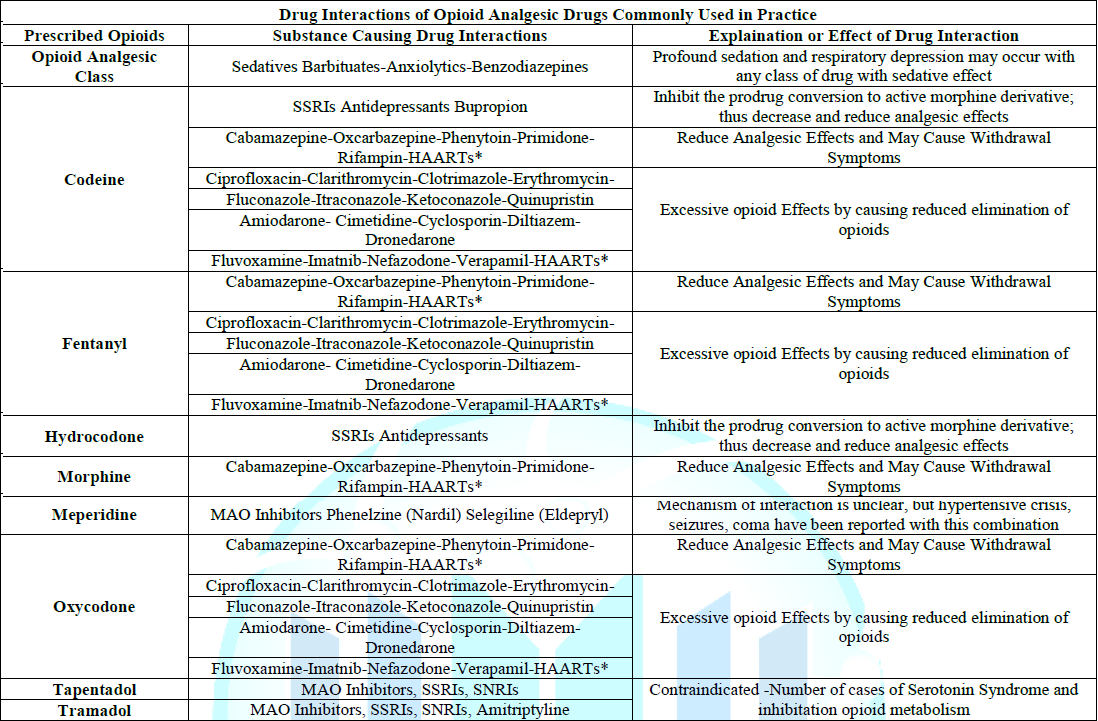
Table 1: Drug Interactions of Opioid Analgesic Drugs Commonly Used in Practice.
Physiological differences between males and females have been observed in plasma volume, body mass index, plasma proteins, body fat, cardiac output, liver blood flow, and hepatic enzyme activity, thus influencing the Hepatic clearance of drugs.
Further, there are known sex differences with all three major renal functions: glomerular filtration, tubular secretion, and tubular reabsorption [17,18]. Morphine has long been considered the gold standard of opioid agents [19]. Morphine has been shown to be more potent and exhibiting a slower onset and offset in women [20,21]. It has been established that women perceive more pain and require greater dosages of morphine to achieve the same antinociceptive effect as in men [21-23].
Offer an explanation for this as higher mu-receptor binding in various cortical and subcortical brain regions exhibited in women than in men, which reveal that women appear to be more sensitive to pain and are more vulnerable to chronic, widespread, and post-procedural pain conditions [24]. Finally, Averitt et al. present evidence that demonstrates a neural basis implicating sex differences in opioid metabolism and neuroimmune signaling with a focus on the periaqueductal gray as a sexual dimorphic core of descending opioid-induced inhibition. They summarize the data to state that both preclinical and clinical research indicate that opioids are less effective in females to explain why women are more likely to be prescribed opioids at higher doses and for longer periods of time than men [25].
Clinical Coping and Opioid Dosing
In the context of opioid stewardship, clinical coping is expending conscious effort to solve potential opioid dosing problems due to the Impact of presenting demographics and disease states that may cause opioid adverse effects, opioid misuse, or opioid abuse disorder, thus seeking to master, minimize, or tolerate these possible issues or conflicts. The effectiveness of this clinical coping efforts depends on the type of opioid issue and/or conflict, the particular individual, and the particular circumstances. Clinical coping suggestions with regard to dosing opioids in the context of demographics and disease states is summarized and presented in Table 2.
In the management of chronic pain in the elderly, physicians should consider a multimodal therapy approach to include nonpharmacologic therapy and non-opioid pharmacologic therapy before initiating opioids [26]. Opioid use should be implemented only when alleviation of pain and improvement of function outweigh the risks to the patient. When selecting non-opioid pharmacologic therapies that may include antidepressants, antiarrhythmic, anticonvulsants, tranquilizers and regional anesthesia, the provider should be aware of the possibility of both drug-drug interactions as well as patient demographic interactions that may result with co-administration of these agents. As always, goals of chronic pain therapy in the elderly are to decrease pain, increase function, and improve overall quality of life. It is recommended that opioids be prescribed at the lowest effective dose, which is approximately 25% to 50% of the starting dose recommended for adult, and then it must be slowly titrated to minimize adverse effects for patients older than age 70 years.The dosage should be reassessed one to four weeks after initiation or dose escalation. Immediate-release formulations of opioids should be initiated before extended-release or long-acting opioids are attempted [27].
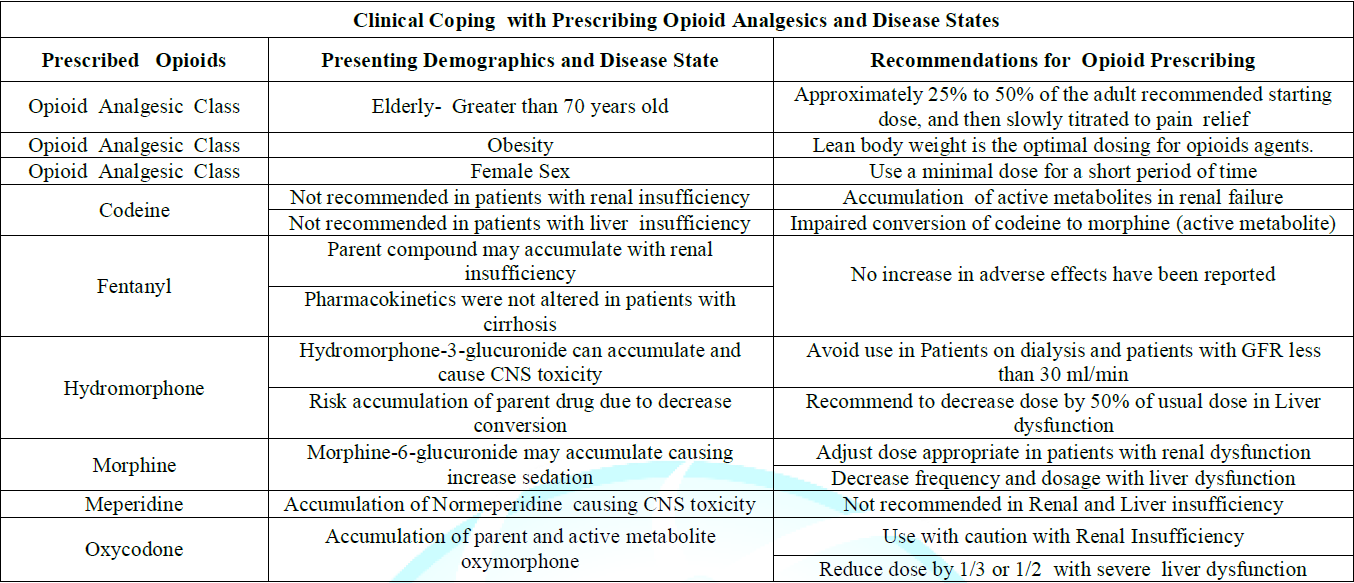
Table 2: Clinical Coping with Prescribing Opioid Analgesics and Disease States.
At the time clinicians prescribe opioids to patients with renal or hepatic dysfunction receive opioid analgesics, it is essential to understand and consider how opioid pharmacokinetics can be altered. This is necessary to ensure appropriate pain relief for the patient while limiting serious and potentially preventable adverse effects, such as respiratory depression, hypotension, or central nervous system toxicity from either the parent drug or its metabolites.
Patients with severe liver disease should be prescribed lower doses of opioids, with extended dosing intervals when multiple daily doses of opioids are needed to relieve pain. Opioids should be used cautiously in patients with severe renal and hepatic dysfunction because of the possible accumulation of the parent drug and/or metabolites.
Usual or adjusted doses may be appropriate for patients prescribed morphine, hydromorphone, and hydrocodone. Oxycodone should not be used in hemodialysis patients, and codeine and meperidine should be avoided at all times. Methadone and fentanyl can be carefully used in patients with renal dysfunction or on dialysis, and methadone is not advised in severe liver failure. For most patients with renal or hepatic dysfunction, either morphine or hydromorphone should be the opioid agents prescribed.
Awareness of the pharmacology of the commonly used opioids is necessary for safe and effective care of morbidly obese patients. Changes in cardiac output and alterations in body composition affect the distribution of numerous opioid drugs. The physician should use a patient’s lean body weight is the most optimal method to dose opioids agents. The increased incidence of obstructive sleep apnea and fat deposition in the pharynx and chest wall places the morbidly obese at increased risk for adverse respiratory events secondary to opioid agents, thus altering the pharmacokinetic and pharmcodynamic properties of opioid agents [10].
Acute pain may often be managed with non-opioid medication. If opioids are used, prescribe the lowest dose for the shortest duration and avoid prescribing refills to reduce the risk for dependency. Literature sources have found that greater use and misuse of prescription medications among older women may be connected to the loss of a partner, low income, mental health issues, or poor overall health. Clinical recommendations for prescribing opioids to women of child-bearing age include the following: assess pregnancy risk in all women of childbearing age prior to prescribing an opioid, avoid prescribing opioids to pregnant women and educate pregnant women about the known risks of opioids to both the mother and the fetus. If opioids must be prescribed to a pregnant woman for acute pain, prescribe the lowest dose and duration appropriate. Provide proper pain control and education to lactating women experiencing acute pain following birth and surgical procedures to avoid opioid adverse effects to the mother and the child [28,29].
A multi-level collaborative health care team approach using an Opioid Stewardship Program (OSP) can provide the necessary frame work to change the current opioid analgesic culture and practice. An OSP can address opioid prescribing, treatment for opioid use disorder, use both educational initiatives and information technology to assist with appropriate opioid prescribing and thus be a helpful tool to curtail the opioid crisis. The acronym “MORPHINE” will be introduced and defined to assist clinicians to appreciate OSPs. The word morphine was selected as an acronym because of its historical significance. Morphine has long been considered the “gold standard” when it comes to pain relief [30-32].
“M” is for multimodal analgesic strategies. A multimodal analgesic approach is likely to produce superior analgesia over the use of an opioid based approach because multimodal analgesic agents target a variety of pain pathways [31-33]. Multimodal analgesic agents target a variety of pain pathways. Many non-opioid multimodal agents to include: heat, ice, massage, physical therapy benefit patients by resulting lower consumption of opioids. It should be acknowledged that opioid medications may provide some measure of relief, but opioids are associated with the health risks of both weight gain and altered glycemic regulation. Further, the use of opioids in a diabetic patient can influence their ability to monitor and control their diabetes by altering their perceptions of activities of daily living to include eating habits, making it more difficult to control your blood glucose levels and the ability to manage your diabetes.
“O” is for the development of an opioid formulary. An opioid formulary identifies those opioids that offer the greatest benefit to a geriatric patient while minimizing risks. Important considerations for objective opioid selection included drug efficiency, safety, patient acceptability, and cost. OSPs can limit opioid initiation by creating prescribing guidelines for providers specializing in treating older patients. Both morphine or fentanyl may be the opioid of choice for an obese or a diabetic patient with careful monitoring. There is always a risk of accumulation of the metabolite of morphine in renal insufficiency [32,33].
“R” is for risk reduction from opioid harm. Therapeutic success depends on proper candidate selection, assessment before administration of opioid therapy, and close patient monitoring. Beyond taking a good medical history via an effective patient interview, there are several risk assessment tools available in OSPs to help identify patients who may be at risk. The clinician should appreciate how the sedative opioid adverse effects may potentiate a sedentary life style that may affect patients with wounds that are potentiated by pressure if a patient cannot off weight them as part of their treatment regimen.
“P” is for pharmacokinetics and pharmacodynamics of opioids. All opioids are metabolized by the liver with age related reduction in CYP3A4 function that affects opioids [34]. Providers must be aware of dangerous combinations of medications, Over-the-counter (OTC) products, and herbal supplements to avoid deadly drug-drug interactions with opioids. Avoid concurrent opioids and benzodiazepines whenever possible [35].
“H” is for help. Pain management specialists can empower a patient’s ability to function and improve their quality of life. Patients who present with complex comorbid pathophysiology as well as multiple drug regimens provide an individual complex challenge for staff and administrators attempting to provide safe and high-quality care for older adults with substance use disorders. Patients with substance use disorders with medically legitimate pain sufficient to justify opioids must be closely monitored [32-33].
“I” is for use of information technology. OSPs can influence electronic records to provide oversight adhering to regulatory changes and evolving state laws that influence prescribing, mandatory prescription drug monitoring program queries, consent for minors for opioid use, and prompts for the initiation of control substance agreements [32].
“N” is for the number of Morphine Milligram Equivalents (MME). Ideally, OSPs can assist geriatric providers to prescribe lower mme amounts to patients by using data collected stored by information technology. Prescribe the lowest effective dosage, carefully reassess benefits and risks when considering increasing dosage to 50 morphine milligram equivalents or more per day [32-34].
“E” is for education to medical professionals, patients, and patient caregivers. It is paramount that an open dialog can be fostered so that expectations of opioid therapy can be appreciated by all parties.31-32 Opioid stewardship principles should become a priority with all opioid prescribers.
Conclusions
Opioid stewardship requires the podiatric physician to acknowledge that opioid pharmacodynamics and pharmacokinetic parameters may be altered in the presence of the following age or disease states: diabetes mellitus, kidney disease, obesity, and sex differences that may impact opioid’s beneficial and possible adverse effects. As a foundation, alterations in opioid pharmacokinetics and pharmacodynamics due to age, illnesses, and sex differences were presented.
Second, building on these clinical alterations in pharmacokinetics and pharmacodynamics were applied to opioid pharmacology to describe possible adverse effects. Finally, methods of clinical coping centered on opioid prescribing were presented and applied to patients who may present with alterations in pharmacokinetics secondary to age, illness, or sex.
References
1. Smith RG. Opioid prescribing: podiatric implications (2018) Podiatry Management 37: 161-169.
2. Smith RG. A review of opioid analgesics frequently prescribed by podiatric physicians (2006) J Am Podiatr Med Assoc 96: 367-373. https://doi.org/10.7547/0960367
3. Campomizzi ME. Pharmacologic management of acute pain: the basics (2004) Pharmacy Practice News 31.
4. Chau DL, Walker V, Pai L and Cho LM. Opiates and elderly: Use and side effects (2008) Clin Interv Aging 3: 273-278.
5. Mangoni AA and Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications (2003) Br J Clin Pharmacol 57: 6-14. https://doi.org/10.1046/j.1365-2125.2003.02007.x
6. Hughes SG. Prescribing for the elderly patient: why do we need to exercise caution? (1998) Br J Clin Pharmacol 46: 531-533. https://doi.org/10.1046/j.1365-2125.1998.00842.x
7. Linnebur SA, O’Connell MB, Wessell AM, McCord AD, Kennedy DH, et al. Pharmacy practice, research, education, and advocacy for older adults (2005) Pharmacotherapy 25: 1404-1405.
8. Tegeder I, Lötsch J and Geisslinger G. Pharmacokinetics of opioids in liver disease (1993) Clin Pharmacokinet. 37: 17-40. https://doi.org/10.2165/00003088-199937010-00002
9. Davies G, Kingswood C and Street M. Pharmacokinetics of opioids in renal dysfunction (1996) Clin Pharmacokinet 31: 410-422. https://doi.org/10.2165/00003088-199631060-00002
10. Ingrande J and Lemmens HJ. Dose adjustment of anaesthetics in the morbidly obese (2010) Br J Anaesth 105: 6-23. https://doi.org/10.1093/bja/aeq312
11. Dostalek M, Akhlaghi F and Puzanovova M. Effect of diabetes mellitus on pharmacokinetic and pharmacodynamic properties of drugs (2012) Clin Pharmacokinet 51: 481-499. https://doi.org/10.1007/bf03261926
12. Gwilt PR, Nahhas RR and Tracewell WG. The effects of diabetes mellitus on pharmacokinetics and pharmacodynamics in humans (1991) Clin Pharmacokinet 20: 447-490. https://doi.org/10.2165/00003088-199120060-00004
13. Ma J, Rayner CK, Jones KL and Horowitz M. Diabetic gastroparesis diagnosis and management (2009) Drugs 69: 971-986. https://doi.org/10.2165/00003495-200969080-00003
14. Benet LZ, Kroetz DL and Sheiner LB. Pharmacokinetics: The dynamics of drug absorption, distribution, and elimination. Hardman JG, Limbird LE (Eds.) (1996) Goodman and Gilman’s The Pharmacological Basis of Therapeutics, McGraw Hill, New York pp. 3-27.
15. Hansten PD and Horn JR. Drug interaction mechanisms: enzyme induction. In: Hansten and Horn’s Drug Interactions Analysis and Management. Facts and Comparison (2003) St. Louis pp. PM1-PM15.
16. Bauer LA. Clinical Pharmacokinetics and pharmacodynamics. Dipro JT (Ed) Pharmacotherapy: A Pathophysiologic Approach (1999) Appleton and Lange, Stamford, USA pp. 21-30.
17. Mallappallil M, Sabu J, Friedman EA and Salifu M. What Do We Know about Opioids and the Kidney? (2017) Int J Mol Sci J 18: 223. https://doi.org/10.3390/ijms18010223
18. Soldin OP and Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics (2009) Clin Pharmacokinet 48: 143-157. https://doi.org/10.2165/00003088-200948030-00001
19. Smith RG. Using clinical-based evidence as the sextant to prescribe and navigate through the opioid crisis (2018) Foot Ankle Quarterly 29: 143-157.
20. Soldin OP, Chung SH and Mattison DR. Sex differences in drug disposition (2011) J Biomed and Biotech 2011: 1-14. https://doi.org/10.1155/2011/187103
21. Sarton E, Olofsen, Romberg R, Hartigh J, Kest B, et al. Sex differences in morphine analgesia: An experimental study in healthy volunteers (2000) Anesthesiology 93: 1245-1254. https://doi.org/10.1097/00000542-200011000-00018
22. Pieretti S, Di Giannuario A, Di Giovannandrea R, Marzoli F, Piccaro G, et al. Gender differences in pain and its relief (2016) Ann Ist Super Sanita 52: 184-189.
23. Craft RM. Modulation of pain by estrogens (2007) Pain 132: 3-12. https://doi.org/10.1016/j.pain.2007.09.028
24. Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B and Riley JL. Sex, gender, and pain: A review of recent clinical and experimental findings (2009) J Pain 10: 447-485. https://doi.org/10.1016/j.jpain.2008.12.001
25. Averitt DL, Eidson LN, Doyle HH and Murphy AZ. Neuronal and glial factors contributing to sex differences in opioid modulation of pain (2019) Neuropsychopharmacology 44: 155-165. https://doi.org/10.1038/s41386-018-0127-4
26. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain (2016) United States, MMWR Recomm Rep 65: 1-49. https://doi.org/10.15585/mmwr.rr6501e1er
27. Koda-Kimble M and Young L. Applied Therapeutics: The Clinical Use of Drugs (2001) Philadelphia, PA: Lippincott Williams and Wilkins
28. Hemsing N, Greaves L, Poole N and Rose Schmidt. Misuse of Prescription Opioid Medication among Women: A Scoping Review (2016) Pain Res Manag. https://doi.org/10.1155/2016/1754195
29. Opioid Use and Opioid Use Disorder in Pregnancy. ACOG Committee Opinion No. 711. American College of Obstetricians and Gynecologists (2017) Gynecol 130: e81-e94. https://doi.org/10.1097/aog.0000000000002235
30. Lapane KL, Quilliam BJ, Chow W and Kim MS. Pharmacologic management of non-cancer pain among nursing home residents (2013) J Pain Symptom Manage 45: 33-42. https://doi.org/10.1016/j.jpainsymman.2011.12.285
31. Sandbrin F and Uppal R. The time for opioid stewardship is now (2019) Jt Comm J Qual Patient Saf 45: 1-2.
32. Perrone J, Weiner SG and Nelson LS. Stewarding recovery from opioid crisis through health system initiatives (2019) West J Emerg Med 20: 198-202. https://doi.org/10.5811/westjem.2018.11.39013
33. Weiner SG, Price CN, Atalay AJ, Harry EM, Pabo EA, et al. A health system-wide initiative to decrease opioid-related morbidity and mortality (2019) Jt Comm J Qual Pat Saf 45: 3-13.
34. Naples JG, Gellad WF and Hanlon JT. The role of opioid analgesics in geriatric pain management (2016) Clin Geriatr Med 32: 725-735. https://doi.org/10.1016/j.cger.2016.06.006
35. Dowell D, Haegerich TM and Chou. CDC Guideline for Prescribing Opioids for Chronic Pain-United States (2016) JAMA 315: 1624-1645. https://doi.org/10.1001/jama.2016.1464
*Corresponding author: Robert G Smith, Shoe String Podiatry, 723 Lucerne Circle, Ormond Beach, Florida 32174, USA, Tel: 386-673-9933, Email: asamaan@cfl.rr.com
Citation
Smith RG. Clinical coping with prescribing opioids within the context of obesity and diabetes mellitus (2019) J Obesity and Diabetes 3: 39-44.
Keywords
Opioids, Diabetes mellitus, Obesity.


 PDF
PDF