A key issue in neurorehabilitation is to improve or restore physical and psychosocial abilities, aiming to maximize activity and participation [1]. In recent years, an innovative rhythm-and-music-based rehabilitation program has successfully been implemented across Europe, as well as in several non-European countries. The Ronnie Gardiner Method (RGM) was created in the 1980s by the Swedish jazz musician Ronnie Gardiner. RGM is now widely used within different settings such as neurological rehabilitation for people with stroke, Parkinsons disease (PD), and multiple sclerosis, as well as in patients with dementia and depression. It is also used in programs targeting healthy aging, ADHD, autism, and dyslexia, and in ordinary school environments. The combination of music and other augmented sensory information, as well as multi-tasking movement exercises, makes it a potentially powerful tool in rehabilitation.
RGM is described as an exercise regimen that challenges motor and cognitive-related abilities by its multi-tasking nature, and is conceptualized as a music-based intervention, i.e., an experimental protocol that uses music in various forms to aid therapeutic effects [2]. Because RGM is a physical activity that is planned, structured, and repetitive, aiming to increase or maintain physical fitness [3], it also meets the criteria for an exercise intervention. The research on the efficacy of RGM is still scarce, but a few scientific trials within the field of neurorehabilitation have thus far shown promising results. A Swedish randomized controlled study on stroke survivors found that the intervention facilitated the participants own perception of recovery, as well as long-lasting improvements concerning balance, grip force, and working memory [4]. Individual interviews were also undertaken with participants to explore personal experiences (submitted manuscript).
Another qualitative study from Sweden found that stroke survivors felt an improved connection to their unfamiliar bodies, and that they felt an improved ability to perform complex movements. The music, the practitioner, and the group were identified as facilitating components [5]. In Parkinsons disease (PD), a small feasibility study found promising results regarding mobility and cognitive function, and the adherence was high, suggesting that the intervention was experienced as enjoyable and motivating [6]. All three studies evaluated RGRM, a former acronym. Our research group is currently exploring the efficacy of RGM in PD regarding cognitive function, balance, fear of falling, and quality of life (ClinicalTrials.gov identification number NCT02999997).
The present article will outline some of the potential effect mechanisms of RGM, using PD as an example. PD is a progressive neurological disease resulting from degeneration of the dopaminergic nigrostriatal pathway of the basal ganglia (Figure 1), and is often accompanied by deficits in executive functions (e.g., attention, processing speed) in addition to motor symptoms such as tremors, rigidity, bradykinesia, and gait and postural difficulties [7]. The number of people with this condition is expected to increase due to the growing aging population [8]. Current medical management is only partially effective in controlling the impairments, and therefore, rehabilitation interventions will play an important role also in the future.
Music-based interventions in general have been found to be beneficial to people with PD [9]. RGM has the potential to alleviate many symptoms of PD: in addition to improving quality of life, specific needs areas that RGM might address are: cognitive function (e.g., attention, spatial cognition, memory, executive function, anticipation, symbol recognition, and speech), motor skills (e.g., gait performance, postural control and body awareness, movement timing, limb coordination, endurance), emotional impact (e.g., enjoyment, mood regulation, self-esteem, reducing depression), and social interaction (facilitating group interaction, reducing social isolation).
One specific problem in PD is an impaired ability of dual-task performance [10], an executive function that is defined as the simultaneous execution of two tasks which have separate goals and often involve motor and/or cognitive tasks [11]. Both motor and cognitive factors may contribute to dual-task deficits in PD, and a number of mechanisms have been suggested to be involved, for example reduced movement automaticity caused by the basal ganglia dysfunction [10].
It has been suggested that rehabilitation strategies that are designed to improve this automatic control of movements have the potential to improve dual-task performance [10]. Improvement of motor-cognitive dual-task performance in individuals with neurologic deficits holds potential for improving gait, balance, and cognition [12]. So far, only few such exercise programs for PD are music-based [13,14]. Based on the integrated multimodal nature of the program, RGM is suggested to specifically improve motor-cognitive dual-task performance in PD.
After a short section describing how RGM is performed, some of the potential effect mechanisms to improve dual-task performance in PD will be outlined. Three specific mediators in RGM are hypothesized to work as mechanisms: the music, external cues, and movement practice (Figure 2).
How it is performed
RGM uses four, for this method
unique, blue and red symbols – resembling hands and feet – that are projected
on a screen, mainly within choreoscores, a form of note systems. The colors
blue and red symbolize the right and left side of the body, respectively. The
symbols can be used alone, or often in pairs. In total there are 19 possible
symbols or combinations.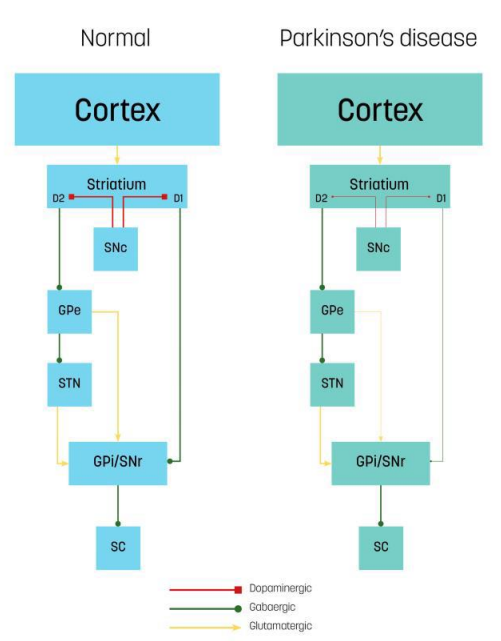
Figure 1: Neural circuits and transmission mechanisms of control in the brains of normal individuals and those with Parkinson’s disease: direct and indirect pathways. The cerebral cortex sends input to the striatum. Dopaminergic projections for the substantia nigra pars compacta (SNc) (red connectors) targets striatal neurons in D1 or D2 receptors. The direct pathway (green connectors): D1 neurons send direct inhibitory projections to the GPi/SNr. The indirect pathway (green and yellow connectors): D2 neurons connect indirectly to the GPi/Snr through the GPe and STN. The SNr inhibits the SC. In Parkinson’s disease, dopaminergic decrease leads to a reduced inhibitory direct pathway output (thin lines) and increased excitatory indirect pathway output (thick lines) onto the GPi/Snr and, consequently, increased SNr inhibition onto the SC as net effect. GPe, external globus pallidus; STN, subthalamic nucleus; GPi, internal globus pallidus; SNr, substantia nigra pars reticulata; SC, superior colliculus
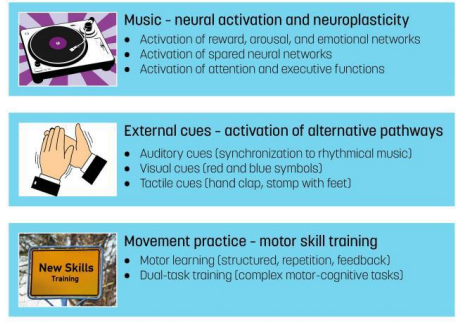
Figure 2:The main potential processes involved in Ronnie Gardiner Method with respect to Parkinson’s disease.
Each one of the 19 symbols is accompanied by a specific movement and a certain four-letter word (e.g., BOOM or CHIC) that is to be pronounced in a loud and clear voice while performing the movement to the sound of rhythmical music. The music (mainly beat-based popular music) in RGM is chosen by the certified RGM practitioner, but efforts are also usually made to use the participants favorite music if possible. The tempo of the performed exercise is measured by the tempo of the music (beats per minutes, BPM). This makes it easy to measure improvement in tempo. For progression, the choreoscores are delivered with increased BPM and with more complex symbols and patterns. The practitioner wears a shirt that is red and blue to reinforce the idea of left and right.
RGM can be delivered in group settings, with the potential benefit of the social interaction, or individually if the participant suffers from fatigue or just needs a quiet environment to recover. To allow for good group dynamics, a recommended group size of 10 to 12 people is suggested. However, to ensure maximal safety, a group size of 6 to 8 individuals may be more appropriate. RGM can be performed in a seated position for people who are unable to stand, or standing up, which is suggested to improve postural stability and stepping ability due to the many weight shifts that activate anticipatory and reactive postural control mechanisms. RGM can be varied in many ways by using varied music, different tempo, and choreoscores with blank spaces to stimulate working memory. There are several short clips available on the video-sharing website YouTube (www.youtube.com) that show its usability.
Theoretical conceptualizations
The rehabilitative effects of music
Music is a powerful tool within neurorehabilitation for enhancing neuroplastic processes in the brain [2,15]. The effects from musical training on training-related plasticity have been extensively investigated [16]. In addition, musical activities induce grey and white matter changes in multiple brain regions, especially in front temporal areas [2]. Music also activates the dopaminergic mesolimbic system of the brain, which regulates memory, attention, executive function, mood, and motivation [2]. Neuroimaging studies have shown that just listening to pleasurable music stimulates dopaminergic regions of the brain, including the nucleus accumbens and ventral tegmental area, which have widespread projections to the cortex [17,18]. This suggests that listening to music stimulates the same networks at those involved in reward and reinforcement learning.
The combination of music and exercise therapy has successfully been used in neurological rehabilitation, especially in PD [19], because music affects many important brain functions. When combined with procedural skill learning (i.e., learning of tasks to be performed with automaticity with little attention or conscious thoughts), the dopaminergic regions responsible for reward, motivation, and learning have the potential to regulate neuroplasticity mechanisms through dopaminergic release and neural synchrony [15]. Learning the complex tasks of RGM is a form of musical training that involves several sensory systems and the motor system, placing demands on a wide variety of higher-order cognitive processes. RGM therefore has the potential to activate the same brain areas as when learning how to play an instrument, although no instruments are involved. For persons with PD, the use of music as therapy has the potential to yield immediate as well as long-term effects that are both motor and cognitive related [20].
Immediate effects: Music-based activities always engage action-related processes in the brain because areas involving rhythm perception are closely linked to those that regulate movement (e.g., cerebellum, premotor cortex, supplementary motor area, and basal ganglia – especially putamen) [17]. People with PD have been shown to benefit from walking to beat-based music. When walking to music, different pathways (externally driven) are used than when walking without this external stimulus (internally driven). For people with PD, the rhythm in music plays a crucial role, as it activates the neural circuits involved in motor actions, replacing the impaired internal timing function. Rhythmical use of musical stimuli thereby enhances audio perception and movement synchronization and compensates for the loss of control by the extrapyramidal system [2].
Long-term effects: Many studies have shown that training with musical rhythm for several weeks can facilitate movement synchronization in persons with PD, improving gait (speed, frequency, and step length), limb coordination, postural control, and balance [20]. Long-term effects on neuroplasticity involve structural changes and remapping of the motor cortex: after several weeks of training (e.g., walking to music), a cortical remapping occurs [15]. With respect to executive functions, it has been proposed that musical training engages the cerebellar-thalamo-cortical network, providing a rerouting to activate executive functions [21]. A music-based intervention that combines both cognitive and physical training, such as RGM, thus has the potential to also improve dual-task performance in people with neurological deficits [22].
Apart from the motor and cognitive effects, music-based activities may reduce anxiety and depression through their impact on reward, arousal, and emotion networks in the brain [2]. The psychological effects and neurobiological mechanisms underlying the effects of music-based interventions are likely to share common neural systems for reward, arousal, affect regulation, learning, and activity-driven plasticity [2].
External cues
External cues are defined as external stimuli (temporal or spatial) that generate an increase in sensory and perceptual sensations to facilitate motor learning and movement initiation [3,23]. External cues provide augmented sensory information and may be visual, somatosensory, or auditory. The benefits of external cues in PD are well known [24,25] and recommended in evidence-based guidelines as a useful rehabilitation tool in PD, especially to improve gait function [3]. Synchronizing movement to external cues facilitates movement initiation, speed, amplitude, and cadence [26,27]. As a result, cues may improve gait during performance of a dual-task by generating rhythm, even in people with mild cognitive impairment [3].
The exact effect mechanisms associated with external cues are still not fully understood [25], but several theories have been brought forward. In PD, the normal internal control of movement is not functioning sufficiently because of the dopamine loss in the basal ganglia. External cues are suggested to replace this reduced internal control [3], supposedly activating cerebellar-thalamic-ventral premotor loops, thereby bypassing the dysfunctional striatum in the basal ganglia [28]. Cues may also act to focus attention, particularly during the performance of more complex tasks [25].
Different external cues work in slightly different ways: visual cues, for example, are suggested to use pathways from the visual cortex and reach motor areas via pontine and cerebellar relays, thereby avoiding the basal ganglia [29]. Auditory cues are suggested to access cortical circuitry (premotor cortex) via the thalamus or cerebellum [30,31]. The perception of beat is an important part of rhythm perception, which has been found to rely on interactions between the auditory and the motor systems [32]. Auditory cues thereby supposedly train attention focus, by interacting with attention oscillators (i.e., internal rhythmic processes) via coupling mechanisms of the brain [33]. Areas involving rhythm perception are closely related to those that regulate movement (premotor cortex, supplementary motor area, cerebellum, and the basal ganglia) [20]. Auditory cues seem to be the most effective cueing strategy [24], and can be delivered by a metronome, although rhythmical music may be preferred [3,24]. These cues have potential for enhancing neural plasticity in that multimodal music training involving senses, movement, and sound has been shown to modulate the auditory cortex [16]. Externally guided tasks with cues can therefore be seen as both compensatory (accessing other parts of the brain) and remediating (enhancing neural plasticity) mechanisms [31,34].
RGM incorporates multiple external cues: somatosensory through body percussion (e.g., handclaps, stomping with feet, slapping thighs), visual (in the form of special symbols displayed on screen), and auditory (beat-based music). This is expected to improve motor control including dual-task performance in PD by the proposed effect mechanisms. In addition, rhythmical entrainment (our inherent tendency to time movements to the regular beat of music) [2] is also trained with RGM. These external cues also have the potential to enhance affective arousal and motivational activation.
Movement practice
The role of exercise to enhance experience-dependent neuroplasticity targeting motor and cognitive circuitry in PD has been emphasized [35]. Movement practice is conceptualized as an update of exercise and entails repetitive motor execution to improve the fluency of motor skills [3]. Two specific areas have been suggested as important interventions in the European physiotherapy guidelines for PD in relation to movement practice: optimizing motor learning and dual-task training [3].
Optimizing motor learning: Motor learning is defined as a set of processes associated with practice of experience leading to relatively permanent changes in the capability for movement [3]. With practice, movement will normally become more efficient with improved interaction between limbs, and more complex movements will be controlled with less effort [36]. The ability to carry out complex motor skills relies much on automaticity [34,37], which depends on intact basal ganglia function. In addition, learning new motor skills involve interactions of the fronto-parietal cortices, the cerebellum, and the basal ganglia (especially the striatum) [3]. In fact, the basal ganglia seem to play a critical role when learning new movements [38]. Because of the basal ganglia deficits, people with PD have an impaired ability to achieve, as well as use, automaticity in daily life [37].
The acquisition of new motor skills is dependent on neuroplastic processes in the brain, including the neurotransmitter dopamine. Because of the low levels of dopamine in PD, it was previously believed that neuroplasticity is diminished in PD. This notion has since been revised [35], and it is now believed that taking into account general principles for motor learning, including the use of augmented sensory information and feedback, will enhance the acquisition of new motor skills and automaticity of movements in PD [3,34,37].
Motor learning is highly dependent on cognitive status in PD. In order to optimize motor learning in PD, treatments should address both the motor deficits and the decreased cortical plasticity [37]. People with PD may require a higher training dose to achieve the same positive results as healthy people [3]. In addition, the effectiveness of motor learning interventions may be improved even further by adding external sensory stimulation [34], such as music in a pleasant social context in an environment that increases enjoyment (i.e., multimodal stimulation) [37]. A frequency of twice weekly sessions for 45 to 50 minutes with a duration of a minimum of 8 weeks is suggested, with additional home exercises to own favorite music if possible.
RGM incorporates multimodal stimulation that is expected to enhance experience-dependent neuroplasticity by targeting both motor and cognitive circuitry. By frequent repetition of the complex movements in RGM in conjunction with various types of enjoyable music, and by using a structured learning schedule, motor learning is expected to be enhanced in PD. Importantly, exercises are typically varied to avoid mental exhaustion by monotonous repetition of the same movements. When performed in a standing position, RGM involves complex motor skill elements such as postural stability with secondary tasks, weight shifting, interlimb coordination, and single leg stance activities. These exercises are expected to improve postural control based on the proposed effect mechanisms.
Dual-task training: It was recently suggested that rehabilitation interventions that combine cognitive training with physical exercises may prove to be the most effective approach to optimize gait in people with PD [39]. Motor-cognitive interventions combine a cognitive with a physical rehabilitation task [40]. Such interventions challenge motor skills, memory, and attention, and are believed to help internally guided task performance, governed by striatal-thalamo-cortical circuits [39]. Exercise programs targeting dual-task ability have been developed for people with PD [41], only few have been music-based. Dual-task interventions may help participants to automate a task, to focus on other tasks, and consequently, to free the processing capacity, whereby more attention is available to process external information [40]. Multi-task exercises (e.g., a combination of motor skill learning, exercise, socialization, and music) are also hypothesized to improve mood and cognition in people with neurological deficits [42].
RGM is a multi-tasking motor-cognitive intervention that targets both cognitive functions such as executive, attention, and visuo-spatial functions, and adds complex rhythmical and reciprocal movements. These movements involve internally cued movements with multi-tasking and attention shifting training with augmented sensory information from external cues. The demands in RGM shift between cognitive/perceptual to motor tasks. Multi-tasking requires that the participants concentrate on and continuously shift attention between the following elements: the instructions given by the practitioner, the specific symbols projected on the screen, the music that is being played, performing the movement that is associated with a certain symbol while pronouncing the correct symbol name, maintaining ones balance (if standing up), the next step to be taken, and not bumping into ones neighbor while performing the exercises. By adding many different tasks, RGM aims at improving the ability to perform a skilled movement with less conscious or executive control or attention directed towards the movement itself, i.e., automaticity, and to improve the ability to switch between tasks.
Clinical implications
Although this work is largely based on research concerning PD, RGM is a useful tool in other fields. RGM is an attractive and enjoyable music-based intervention with many potential benefits. RGM incorporates several components working as potential effect mechanisms including rhythmical beat-based music visual and auditory cues sensory stimulation (body percussion, tactile cues) challenging cognitive tasks and body movements such as weight shifting. Furthermore, RGM promotes positive experiences that may enhance functional improvements, mental engagement, motivation, and well-being. RGM is relatively non-strenuous and does not require high physical capacity, which makes it a participant-friendly rehabilitation method. RGM does, however, not specifically target muscle strength, although movements such as standing up and sitting down are incorporated during practice. RGM suits many clinical settings, and the certified practitioners can have various professional backgrounds: music therapists, physical therapists, occupational therapists, exercise and movement therapists, speech therapists, or dance instructors.
Conclusion
RGM is an innovative music-based intervention with the potential to improve several aspects in people with neurological deficits. This article has outlined the theoretical background with respect to Parkinsons disease however, RGM can be used for any condition. Because of the novelty of RGM, the evidence for the effectiveness is still scarce, and there is a need to evaluate RGM in clinical trials in different settings and in different conditions.
Acknowledgements
The author wishes to thank Mariken Jaspers, RGM Nederland, for checking the accuracy of the description of RGM. This work was financially supported by the County Council of Östergötland, Tornspiran Foundation, Neuro Sweden, Henry and Ella Margareta Ståhls Foundation, and Research Foundation for Parkinsons disease. The Funders had no role in study design, data collection, decision to publish, or preparation of the manuscript.
References
1.
Khan F, Amatya B, Galea MP,
Gonzenbach R, Kesselring J. Neurorehabilitation: Applied neuroplasticity (2017)
J Neurol 264:603-615.
2.
Sihvonen AJ, Särkämö T, Leo V,
Tervaniemi M, Altenmüller E et al. Music-based interventions in neurological
rehabilitation (2017) Lancet Neurol 16:648-660.
3.
Keus S, Munneke M, Graziano M,
Paltamaa J, Pelosin E, et al. European physiotherapy guideline for Parkinsons
disease: Development and Implementation (2014) Movement Disord 29:537.
4.
Bunketorp-Käll L, Lundgren-Nilsson
Å, Samuelsson H, Pekny T, Blomvé K, et al. Long-Term Improvements After
Multimodal Rehabilitation in Late Phase After Stroke: A Randomized Controlled
Trial (2017) Stroke 48:1916-1924.
5.
Thornberg K, Josephsson S and
Lindquist I. Experiences of participation in rhythm and movement therapy after
stroke (2014) Disabil Rehabil 36:1869-1874.
6.
Pohl P, Dizdar N and Hallert E.
The Ronnie Gardiner Rhythm and Music Method - A feasibility study in Parkinsons
disease (2013) Disabil Rehabil 35:2197-2204.
7.
Miura K, Matsui M, Takashima S and
Tanaka K. Neuropsychological Characteristics and Their Association with Higher-Level
Functional Capacity in Parkinsons Disease (2015) Dement Geriatr Cogn Dis Extra 5:271-284.
8.
Dorsey ER and Bloem BR. The Parkinson
Pandemic-A Call to Action (2018) JAMA Neurol 75:9-10.
9.
Zhang S, Liu D, Ye D, Li H and
Chen F. Can music-based movement therapy improve motor dysfunction in patients
with Parkinsons disease? Systematic review and meta-analysis (2017) Neurol Sci
38:1629-36.
10. Kelly
VE, Eusterbrock AJ and Shumway-Cook A. A review of dual-task walking deficits in
people with Parkinsons disease: motor and cognitive contributions, mechanisms, and
clinical implication (2012) Parkinsons Dis 918719.
11. McIsaac
TL, Lamberg EM and Muratori LM. Building a framework for dual task taxonomy (2015)
Biomed Res Int 591475.
12. Fritz
NE, Cheek FM and Nichols-Larsen DS. Motor-Cognitive Dual-Task Training in
Persons with Neurologic Disorders: A Systematic Review (2015) J Neurol Phys
Ther 39:142-153.
13. Brown
LA, de Bruin N, Doan JB, Suchowersky O and Hu B. Novel challenges to gait in Parkinsons
disease: The effect of concurrent music in single- and dual-task contexts (2009)
Arch Phys Med Rehabil 90:1578-1583.
14. de
Bruin N, Doan JB, Turnbull G, Suchowersky O, Bonfield S et al. Walking with
music is a safe and viable tool for gait training in Parkinsons disease: The
effect of a 13-week feasibility study on single and dual task walking (2010)
Parkinsons Dis 483530.
15. Stegemöller
EL. Exploring a neuroplasticity model of music therapy (2014) J Music Ther 51:211-227.
16. Lappe
C, Trainor LJ, Herholz SC and Pantev C. Cortical plasticity induced by
short-term multimodal musical rhythm training (2011) PLoS One 6:e21493.
17. Koelsch
S. A neuroscientific perspective on music therapy (2009) Ann N Y Acad Sci
1169:374-84.
18. Zatorre
RJ and Salimpoor VN. From perception to pleasure: Music and its neural
substrates (2013) Proc Natl Acad Sci U S 110:10430-10437.
19. de
Dreu MJ, van der Wilk AS, Poppe E, Kwakkel G and van Wegen EE. Rehabilitation,
exercise therapy and music in patients with Parkinsons disease: A meta-analysis
of the effects of music-based movement therapy on walking ability, balance and
quality of life (2012) Parkinsonism Relat Disord 1:114-119.
20. Raglio
A. Music Therapy Interventions in Parkinsons Disease: The State-of-the-Art (2015)
Front Neurol 6:185.
21. Lesiuk
T, Bugos JA and Murakami B. A Rationale for Music Training to Enhance Executive
Functions in Parkinsons Disease: An Overview of the Problem (2018) Healthcare
(Basel) 6.
22. Chen
YL and Pei YC. Musical dual-task training in patients with mild-to-moderate
dementia: A randomized controlled trial (2018) Neuropsychiatr Dis Treat 14:1381-1393.
23. Rocha
PA, Porfírio GM, Ferraz HB and Trevisani VF. Effects of external cues on gait
parameters of Parkinsons disease patients: A systematic review (2014) Clin
Neurol Neurosurg 124:127-134.
24. Spaulding
SJ, Barber B, Colby M, Cormack B, Mick et al. Cueing and gait improvement among
people with Parkinsons disease: A meta-analysis (2013) Arch Phys Med Rehabil 94:562-570.
25. Peterson
DS and Smulders K. Cues and Attention in Parkinsonian Gait: Potential Mechanisms
and Future Directions (2015) Front Neurol. 6:255.
26. Rochester
L, Hetherington V, Jones D, Nieuwboer A, Willems AM et al. The effect of
external rhythmic cues (auditory and visual) on walking during a functional
task in homes of people with Parkinsons disease (2005) Arch Phys Med Rehabil 86:999-1006.
27. Dibble
LE, Nicholson DE, Shultz B, MacWilliams BA, Marcus RL et al. Sensory cueing
effects on maximal speed gait initiation in persons with Parkinsons disease and
healthy elders (2014) Gait Posture 19:215-225.
28. Hackney
ME, Lee HL, Battisto J, Crosson B and McGregor KM. Context-Dependent Neural
Activation: Internally and Externally Guided Rhythmic Lower Limb Movement in
Individuals With and Without Neurodegenerative Disease (2015) Front Neurol
6:251.
29. Cerasa
A, Hagberg GE, Peppe A, Bianciardi M, Gioia MC et al. Functional changes in the
activity of cerebellum and frontostriatal regions during externally and
internally timed movement in Parkinsons disease (2006) Brain Res 71:259-269.
30. Nieuwboer
A, Feys P, de Weerdt W and Dom R. Is using a cue the clue to the treatment of
freezing in Parkinsons disease (1997) Physiother Res Int 2:125-132.
31. Chuma
T, Faruque Reza M, Ikoma K and Mano Y. Motor learning of hands with auditory
cue in patients with Parkinsons disease (2006) J Neural Transm 113:175-185.
32. Grahn
JA. Neural mechanisms of rhythm perception: current findings and future perspectives
(2012) Top Cogn Sci 4:585-606.
33. Thaut
MH, McIntosh GC and Hoemberg V. Neurobiological foundations of neurologic music
therapy: Rhythmic entrainment and the motor system (2014) Front Psychol 5:1185.
34. Nieuwboer
A, Rochester L, Müncks L and Swinnen SP. Motor learning in Parkinsons disease: Limitations
and potential for rehabilitation (2009) Parkinsonism Relat Disord 3:53-58.
35. Petzinger
GM, Fisher BE, McEwen S, Beeler JA, Walsh JP et al. Exercise-enhanced
neuroplasticity targeting motor and cognitive circuitry in Parkinsons disease (2013)
Lancet Neurol 12:716-726.
36. Altenmüller
E and Schlaug G. Apollos gift: New aspects of neurologic music therapy (2015)
Prog Brain Res 217:237-252.
37. Marinelli
L, Quartarone A, Hallett M, Frazzitta G and Ghilardi MF. The many facets of
motor learning and their relevance for Parkinsons disease (2017) Clin
Neurophysiol 128:1127-1141.
38. Krebs
HI, Hogan N, Hening W, Adamovich SV and Poizner H. Procedural motor learning in
Parkinsons disease (2001) Exp Brain Res 141:425-437.
39. Salazar
RD, Ren X, Ellis TD, Toraif N, Barthelemy OJ et al. Dual tasking in Parkinsons
disease: Cognitive consequences while walking (2017) Neuropsychology
31:613-623.
40. Pichierri
G, Wolf P, Murer K and de Bruin ED. Cognitive and cognitive-motor interventions
affecting physical functioning: A systematic review (2011) BMC Geriatr 11:29.
41. Yogev-Seligmann
G, Giladi N, Brozgol M and Hausdorff JM. A training program to improve gait
while dual tasking in patients with Parkinsons disease: A pilot study (2012)
Arch Phys Med Rehabil 93:176-181.
42. Dhami
P,Moreno S and DeSouza JF. New framework for rehabilitation - fusion of
cognitive and physical rehabilitation: The hope for dancing (2014) Front
Psychol 5:1478.
Keywords
Parkinsons disease, Neuroplasticity, Multiple sclerosis, Stroke, Autism,Dementia,Dyslexia


 PDF
PDF