Commentary :
A clot that obstructs blood flow triggers the onset of most heart
attacks and 85% of strokes,
the leading causes of death and disability worldwide. Reestablishing blood flow rapidly limits
damage to the heart or brain, saves lives, and can restore well-being. Since “time is heart or brain,” treatment
must be initiated rapidly, which means by a readily available method. Fibrinolysis
is the only therapy able to fulfill these criteria but the current method using
tissue
Plasminogen Activator (tPA) monotherapy is not sufficiently safe to be
administered without pre-testing nor is it sufficiently effective to be very
useful. Although anticoagulants
have evolved, especially in recent years, the same thrombolytic has been used
since 1987, when tPA monotherapy was approved for the treatment of Acute
Myocardial Infarction (AMI) and subsequently also for ischemic
stroke. However, the clinical results with tPA given within the time
window was never shown to be significantly better than those obtained with the
previous thrombolytic, Streptokinase (SK) [1], moreover tPA caused more
bleeding side effects than SK. When tPA is used for ischemic stroke there is a 6-7% incidence of symptomatic
intracranial hemorrhage complications [2].
As a result, tPA has recently been replaced, not by another thrombolytic,
but by Percutaneous
Coronary Intervention (PCI), a method that is costly and
time-consuming. Nevertheless, the
results of PCI were superior to those obtained with tPA. In stroke, tPA is used in only about 5% of
patients because of its limited efficacy and hazard. tPA is part of the bodys natural thrombolytic system that
controls the size of “good” blood clots needed to stop bleeding, and which is
also needed for the repair of “wear and tear” injuries of blood vessels. In this system, tPA is remarkably effective,
in contrast to its relatively poor efficacy in therapy. This discrepancy is explained by the presence
of the other thrombolytic, urokinase
Plasminogen Activator or uPA, in the natural system. In the biological system, tPA is found in the vessel wall,
and in the event of an obstructive thrombus, it is released at that site. Due to its high fibrin affinity, mediated by
both its finger and kringle domains, tPA binds to the fibrin clot, and
activates an adjacent fibrin-bound plasminogen [3] thereby initiating
fibrinolysis. This fibrin degradation
exposes two new plasminogen binding sites on the fibrin surface. Since tPA has only a single fibrin binding
site on the fibrin D-domain, it cannot activate the plasminogen on these new
sites which are on the fibrin E-domain.
Only at very high doses, at which tPA is no longer fibrin-specific, can
tPA activate these new plasminogens.
Instead, the first of these new plasminogens is activated not by tPA but
rather by prourokinase
(proUK), which has a high substrate affinity for plasminogen on the fibrin
E-domain. Activation of this plasminogen
is followed by the reciprocal activation of proUK to UK by plasmin. The UK then activates the remaining
plasminogen completing fibrinolysis [3].
Therefore, fibrinolysis involves the sequential effects of
both plasminogen activators with tPA initiation of lysis and proUK/UK
continuing and completing it. As a
result, tPA is responsible for 33% of the process and proUK/UK the remaining
66%. The dominant role of proUK in
fibrinolysis has been generally over looked, which may be explained by the fact
that most of it is carried on the surface of platelets to which proUK is
tightly bound and where it is
fibrinolytically active [4].
Unfortunately, since proUK was ignored, therapeutic fibrinolysis has
consisted of tPA alone for the past 32 years.
However, since tPAs function is limited to the initiation of
fibrinolysis, its therapeutic effect has always been a disappointment being
somewhat analogous to trying to run a car on the starter motor alone. At the same time, the complementary fibrinolytic properties
of tPA and proUK show that in biology they were intended to function in
combination. When used alone to dissolve
a clot they are inefficient and requiring high, non-specific doses, whereas in
combination low, fibrin-specific doses can be used since their combined effects
are synergistic. For example, small
doses of tPA and proUK that induce 25% clot lysis by each alone, induce 100%
lysis when they are combined (Figure 1).
1.
Brophy JM and Joseph L. Placing
trials in context using Bayesian analysis.
GUSTO revisited by reverend Bayes (1995) JAMA 273: 871-875. 2.
IST-3 Collaboration Group. The benefits and harms of intravenous
thrombolysis with recombinant tissue plasminogen activator within 6 h of acute
ischemic stroke: a randomized control trial (2012) Lancet 379: 2352-2363. https://doi.org/10.1016/S0140-6736(12)60768-5 3.
Pannell R, Li S and Gurewich
V. Highly
effective fibrinolysis by a sequential, synergistic combination of mini-dose
tPA plus low dose proUK (2015) PLOS One 10: 1-15. 4.
Rijken DC, Hoylaerts M and Collen
D. Fibrinolytic properties of one-chain and two-chain (human) tissue type
plasminogen activator (1982) J Biol Chem 257: 2920-2925. 5.
Gurewich V, Johnstone M, Loza JP
and Pannell R. Prourokinase and prekallikrein are both associated with
platelets. Implications for the
intrinsic pathway of fibrinolysis and for therapeutic thrombolysis (1993) FEBS
Lett 318: 317-321. http://dx.doi.org/10.1016/0014-5793(93)80537-5 6.
Zarich SW, Kowalchuk GJ, Weaver
WD, Loscalzo J, Sassower M, et al. Sequential combination of thrombolytic
therapy for acute myocardial infarction: results of the Pro-Urokinase and tPA
enhancement of thrombolysis (PATENT trial) (1995) J Am Coll Cardiol 26: 374-379. http://dx.doi.org/10.1016/0735-1097(95)80009-6 7.
GUSTO Angiographic investigators. The effects of tissue plasminogen activator,
streptokinase or both on coronary patency and survival after acute myocardial infarction
(1993) New Eng J Med 329: 1615-1622.
Effective and Safe Fibrinolysis Requires both Plasminogen Activators in a Sequential Combination
Full-Text
The Fibrinolytic
System
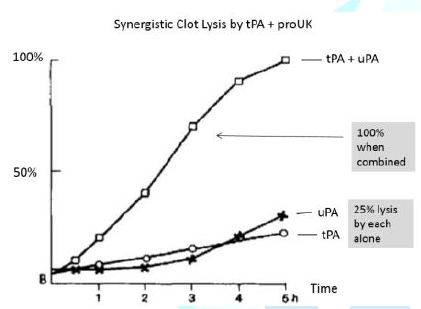
Figure 1: Lysis by tPA/uPA alone or in combination.
A similar low-dose combination was tested clinically in 101 patients
with AMI who were given a 5 mg bolus dose of tPA followed by an infusion of 40
mg/h of proUK for 90 min. The coronary
vessel responsible for the attack was opened in 82% of the patients, and there
was only one death and no bleeding complications [5] (Figure 2). These unprecedented results are a substantial
improvement over GUSTO, the best of the tPA trials, in which the coronary
responsible for the AMI was opened in only 45% of patients, and deaths were
6.3%. These results represent a clinical verification of in vitro
studies tPA and proUK and show that effective and safe fibrinolysis requires
both of these natural plasminogen activators in a sequential combination, and
that monotherapy with tPA alone has been an unfortunate mistake.References