Introduction
Diabetes has
been recently more prevalent across the world, associated with several diseased
states. They include obesity, metabolic syndrome, Non-Communicable Diseases (NCDs),
liver diseases, and so on [1]. As to the direction of diabetic treatment,
optimal therapy of T2DM would include patient-oriented goals associated with
normalized glucose variability, and minimized influences of hypoglycemia and
weight gain, and reduced risk of macroangiopathy and microangiopathy as
diabetic complications [2].
Regarding the treatment for diabetes, the basic principle is nutritional therapy. There has been Calorie Restriction (CR), which was formerly standard diet therapy. After that, Low Carbohydrate Diet (LCD) was introduced to medical practice and health care area [3]. The evidence of clinical efficacy of LCD was presented by several researchers [4,5]. Authors and collaborators have continued developing LCD for several opportunities medically and socially [6]. For useful method in the daily life, three kinds of LCD were proposed broadly, which are super-LCD, standard-LCD and petite-LCD [7]. We have published textbooks of LCD, prepared workshops and seminars for LCD through the activity of Japan LCD Promotion Association (JLCDPA) [8]. Furthermore, various diabetic research has continued concerning CR, LCD, Meal Tolerance Test (MTT), Continuous Glucose Monitoring (CGM), and so on [9]. For pharmacological diabetic options, rather new foci include the clinical application of several kinds of Glucagon like Peptide-1 Receptor Agonists (GLP-1RAs) [10]. GLP-1RAs include liraglutide, exenatide, lixisenatide, dulaglutide and others. Recently, a Network Meta-Analysis (NMA) for GLP-1RAs were conducted for evaluating glycemic control and safety outcomes [11]. The research included 23209 cases with 18 GLP1-RA regimens from 54 studies, concluded the efficacy of glycemic control and cardiometabolic benefits, and recommended patient-oriented clinical decisions for the consideration of comparative profiles.
Among
several GLP-1RAs, liraglutide has been rather widely used in medical practice.
Related to liraglutide, Xultophy has been introduced to diabetic treatment,
which is the combination of liraglutide and insulin degludec [12]. In
comparison with GLP-1RA, the combined agent was evaluated to show clinical
beneficial effect [13]. GLP-1RA can decrease Fasting Plasma Glucose (FPG) and
reduce the post-prandial response of blood glucose [14]. Furthermore, basal
insulin can decrease FPG, and the combination of the fixed ratio can control
the diabetic situation with simple and useful manner. Consequently, Xultophy
will improve the function of beta cell and also maintain the cardioprotective
activity [15]. As a matter of fact, Xultophy has been evaluated for its
beneficial effect and been gradually more used. Authors have continued diabetic
treatment for T2DM patients with various background and complications. We have
experienced two diabetic cases with impressive clinical progress treated by
Xultophy. Their general outlines with some discussion will be presented in this
article.
Presentation of Case 1
History and Physicals: The patient is a 59-year-old male,
who has been treated hypertension for 20 years. He was pointed out
hyperglycemia with HbA1c 10% in 2018, and has been treated by insulin with
Novolin 30R twice a day. He felt sensory disturbance and slight motor abnormality
in the left extremities in January 2020, and was admitted for further
evaluation and treatment. He was diagnosed as Cerebral Vascular Accident (CVA)
in subtle degree. However, head Computed Tomography (CT) scan did not show the
clear evidence of CVA. Several laboratory data in January 2020 were as follows.
HbA1c 9.0%, pre-prandial glucose 182 mg/dL, TP 6.9 g/dL, Alb 4.3 g/dL, AST 17
U/L, ALT 15 U/L, g-GT 79 U/L, uric acid 4.3 mg/dL, BUN 13 mg/dL, Cr 1.0 mg/dL,
eGFR 61 mL/min/1.73m2, LDL-C 118 mg/dL, HDL 50 mg/dL, TG 241 mg/dL,
Hb 15.1 g/dL, RBC 483 x 106 /μL, WBC 7200 /μL, Plt 26.6 x 104
/μL, CRP 0.06 mg/dL.
Clinical Progress
Successively, he has been treated hypertension, CVA and T2DM and received rehabilitation of sensory disturbance in subtle degree. He has only felt some numbness in left hand and leg, and not show any clear symptoms or signs of left hemiparesis, speech disturbance, or other motor disturbance. As to the treatment, he was given Nifedipine CR 20mg 1Tab, Clopidgrel 75mg 1Tab, Linagliptin 5mg 1Tab, and Novolin 30R twice in the morning and evening. The changes in HbA1c and treatment of T2DM are shown in (Figure 1). Regarding insulin treatment, Novolin 30R has been provided at 14-30 units in the morning and 8-21 units in the evening. HbA1c value has been unstable ranging from 7.3% to 11.3%. For the improvement of glucose variability, the treatment was changed from insulin to Xultophy in December, 2020.
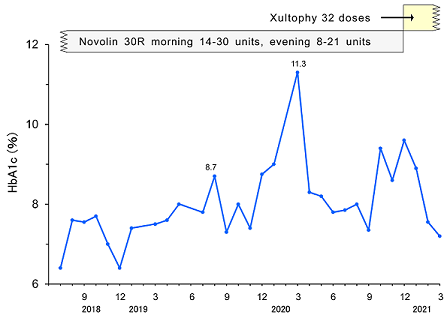
Figure 1: Clinical progress of Case 1.
The detail progress of provided doses was summarized in (Table 1). It shows gradual increase doses of Xultophy and responsive glucose decrease for about 2 weeks. When Xultophy was provided up to 32 doses, the profile of blood glucose was improved at satisfactory level. Consequently, case 1 seems to show successful efficacy of Xultophy for better control of blood glucose variability.
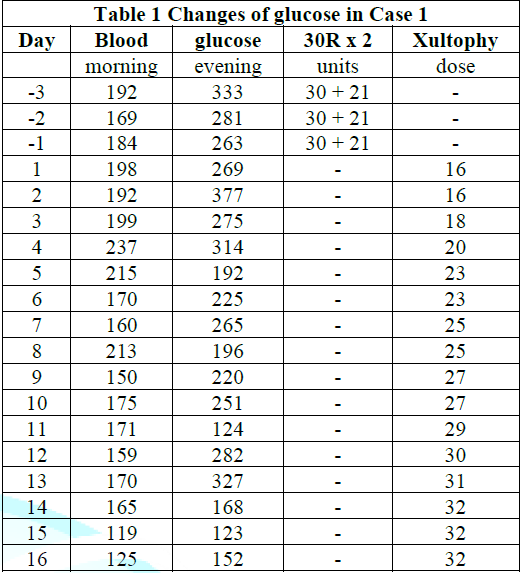
Table 1: Changes in blood glucose in Case 1.
Presentation of Case 2
History and Physicals: The patient is a 78-year-old male,
who has multiple medical problems and complications. T2DM was pointed out at
the age of 54, and Oral Hypoglycemic Agents (OHAs) were provided for long
years. After that, he was suffered from hypertension, occasional bronchial
asthma, and diabetic nephropathy at 3rd stage, hyperuricemia, reflux
esophagitis associated with various treatments. He had been also a heavy
drinker taking about 80g of pure alcohol on average per day for 50 years. In
recent 2 years, additional problem included pneumonia, adhesive ileus, and
Alzheimer dementia and so on. As to the treatment of T2DM, he received
gliclazide and pioglitazone in 1996, glimepiride and pioglitazone in 2008,
Revemil (insulin detemir 5 units/day) in 2009, Victoza (liraglutide 18mg/day)
in 2011 and Byetta (exenatide 10μg/ day) in 2013. He developed unstable
condition associated with general malaise, heartburn, diarrhea and other
symptoms in the autumn of 2019. Then, he was admitted for further evaluation
and treatment.
Physicals
showed no remarkable abnormalities of vitals, consciousness, chest, abdomen and
neurological findings. Laboratory data were in the following. Hb 13.0 g/dL, RBC
413 x 106 /μL, WBC 3500 /μL, Plt 16.2 x 104 /μL, TP 6.8
g/dL, Alb 3.6 g/dL, BUN 26 mg/dL, Cr 1.2 mg/dL, uric acid 5.9 mg/dL, eGFR 47
mL/min/1.73m2, TG 111 mg/dL, HDL-C 41 mg/dL, LDL-C 110 mg/dL, AST 26
U/L, ALT 28 U/L, g-GT 29 U/L, urine protein 0.31 g/gCr (-0.15). Several exams
included that no retinopathy in fundus, nerve conduction velocity within normal
limits, the coefficients of variation of RR intervals (CVRR) 1.95%, abdominal
echo showed chronic liver disease and hemangioma in the segment 5 (10 x 7 x
10mm), carotid artery showed plaque bilaterally, cardiac echography is
unremarkable, ankle brachial index (ABI) 1.13/1.19. cardio-ankle vascular index
(CAVI) 10.0/10.0.
Clinical Progress
After the evaluation of diabetic exam and general condition, the treatment for T2DM was changed into Tresiba (Degludec) 6 units and NovoRapid 16-8-8 units (Figure 2). Within a few months, the therapeutic amount was changed to 8-10 units and 8-8-8 units. In November and December, 2020, HbA1c was elevated. Although the treatment doses of Degludec and NovoRapid were increased, control of glucose variability was not enough. Consequently, the administration of Xultophy (IDegLira) was started, which include degludec and liraglutide.
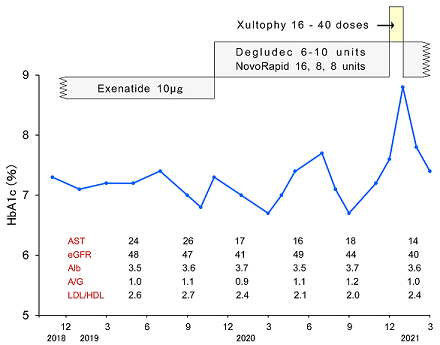
Figure 2: Clinical progress of Case 2 with the changes of treatment and laboratory data.
The detail progress of provided doses starting 16 doses was summarized in (Table 2). The amount of Xultophy was rapidly increased up to 40 doses. His blood glucose was rather decreased in the morning, but it persisted rather higher in the evening. This treatment was continued about 2 weeks, and then patient came to complain of general malaise, nausea, thirsty, polydipsia and polyuria in the afternoon and evening. Those symptoms became rather exacerbated, where this treatment was evaluated to be not tolerated. Consequently, the treatment was changed back to the previous method with Degludec and Novorapid. Just after the same units of them started again, the blood glucose showed rather controlled level. From mentioned above, Xultophy seemed to be not effective in this case.
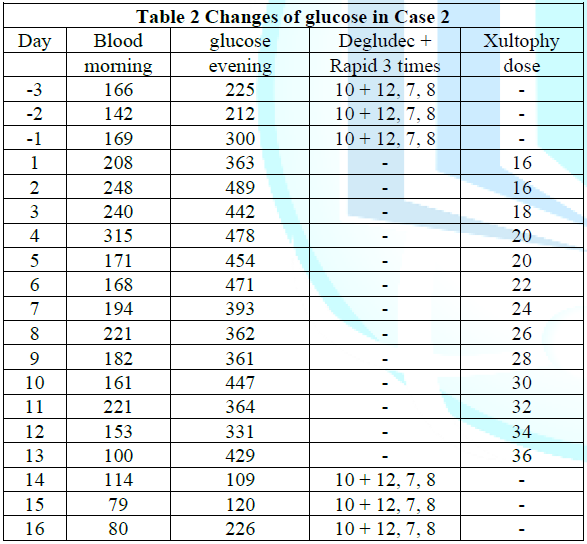
Table 2: Changes in blood glucose in Case 2.
Discussion
For standard
method of diabetic treatment, T2DM patients are firstly started with oral
hypoglycemic agents (OHAs) and then insulin will be provided if necessary. In
order to control blood glucose as finely as possible, treatment was formerly basal-bolus
injection regimen [16]. In other words, Multiple Daily Insulin Injection (MDI)
was rather standard and prevalent. However, recent situation shows that
once-daily injection has been increasing. One of the reasons would be
increasing clinical application of Xultophy, which is evaluated as useful,
convenient and effective treatment regimen [17]. A series of the investigation
of clinical response of Xultophy have been found, which is the European
Xultophy Treatment Retrospective Audit (EXTRA) study. From European diabetes
centers, a Real-Word Evidence (RWE) study (EXTRA) was reported [18]. The result
showed a significant decrease of HbA1c value (-0.7%) and also of weight (-2.4
kg) for 6 months in the subjects who changed from MDI to Xultophy. Related to
EXTRA study, large investigation included 611 cases from 5 countries. As a
result, the initiation therapy showed substantial -0.9% reduction of HbA1c
(p<0.001) after six months [19]. Consequently, Xultophy seems to be
beneficial and convenient agent for T2DM associated with adequate continuation
of regular lifestyle. In this article, two cases treated with Xultophy were
presented. Regarding case 1, HbA1c value was formerly unstable from 6.2% to
11.3%. For insulin treatment, Novolin 30R was administered twice in the morning
and evening [20].
The
treatment was switched to Zultophy in order to improve the daily profile of
blood glucose. After starting 16 doses, blood glucose levels were gradually
decreased, and became stable at 32 doses of Xultophy. He felt formerly large
daily glucose fluctuations from day to day, but after the change to Xultophy,
blood glucose fluctuations became smaller and stabilized. It seems to be
beneficial because it has the simple advantage of injection once a day,
stabilizes daily blood glucose level, improves HbA1c, and has unremarkable
adverse effects [21]. The case 2 showed characteristic point concerning
glycemic variability. Fasting Plasma Glucose (FPG) in early morning has been
almost in satisfactory range. On the other hand, FPG in the evening has been
significantly elevated. In response to this situation, the dose of Xultophy was
increased, but hyperglycemia in the evening did not improve. One of the reasons
would be from the medical history that he had been a heavy drinker for 50 years
[22].
Furthermore,
the results of several examination may suggest the existence of Chronic Liver
Dysfunction (CLD), including AST, Alb, A/G ratio and image data. For CLD
situation, the function of converting glucose into glycogen is reduced in the
liver, as well as reduced restoring function [23]. Then, the excess glucose may
flow into the blood directly, resulting in abrupt glucose increase after meal.
It brings post-prandial hyperglycemia. Furthermore, blood glucose in early
morning is kept relatively low in this case. For CLD, gluconeogenesis in the
liver is reduced, and then blood glucose tends to be lower on early morning
fasting [24].
This may be
the reason why this case shows rather lower blood glucose in early morning. Case
2 was formerly given exenatide for some period. He felt general malaise and
discomfort in the abdomen after starting liraglutide in Xultophy. It may be
involved in the adverse effect of GLP-1RA [25]. Gastrointestinal (GI) adverse
events (AEs) have been found in GLP-1RAs. There were studies of GI-AEs for
semaglutide vs other GLP-1RAs [26]. They include SUSTAIN (vs exenatide),
SUSTAIN 7 (vs dulaglutide) and SUSTAIN 10 (liraglutide). GI-AEs symptoms show
diarrhea, constipation and dyspepsia during dose escalation. Another study was
comparative study of once-weekly semaglutide and once-daily liraglutide [27].
Consequently, unstable condition of the case might be related with the given
liraglutide as GLP-1RA.
On the other hand, GLP-1RAs have beneficial efficacy for liver function or diseased state of fatty liver with diabetes. Among GLP-1RAs, liraglutide was investigated for the interaction of liver function in CLD patients [28]. Liver enzymes were not influenced for 2 years by this agent alone or combined with others. Liraglutide showed clinical efficacy associated with pioglitazone or sitagliptin for patients with diabetes and Non-Alcoholic Fatty Liver Disease (NAFLD). Liraglutide has reduced diabetic parameters, body weight, inflammatory situation and liver fibrosis [29]. Recently, there was an impressive study that circulating levels of GLP-1 were compared between cirrhotic patients and healthy controls [30]. As a result, GLP-1 value was 95.3 vs 111.8 pg/mL (p=0.017), respectively. Further prospective investigation will be necessary with exploring the efficacy of incretion.
In conclusion, two cases treated with Xultophy were described with some perspectives. In actual practice for diabetes, Xultophy has been gradually used more because of its satisfactory clinical efficacy of decreased glucose and HbA1c values and unremarkable adverse effect. However, however, clinical responses are not always satisfactory due to different complication and background of each patient. In this report, there are some limitations. Two cases have different background, complications and T2DM conditions. Xultophy was effective in case 1, but not effective in case 2. One of the less responsiveness to Xultophy would be due to impaired liver function. Clinical progress of both cases associated with several perspectives from various points of view are discussed in this article. This report may become one of the reference data for various interrelationship among diabetes, liver function, endocrine and gastroenterological axes.
References
- 2 Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2021(2021) American Diabetes Association 44: S15-S33. https://doi.org/10.2337/dc21-s002
- Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) (2018) Diabetologia 61: 2461-2498. https://doi.org/10.1007/s00125-018-4729-5
- Atkins RC. Dr Atkins' New Diet Revolution (2009) Harper.
- Yokose C, McCormick N, Rai KS, Lu N, Curhan G, Schwarzfuchs D, et al. Effects of Low-Fat, Mediterranean, or Low-Carbohydrate Weight Loss Diets on Serum Urate and Cardiometabolic Risk Factors - A Secondary Analysis of the Dietary Intervention Randomized Controlled Trial (DIRECT) (2020) N Engl J Med 359: 229-241. https://doi.org/10.2337/figshare.12780059.v1
- Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base (2015) Nutrition 31: 1-13. https://doi.org/10.1016/j.nut.2014.06.011
- Ebe K, Ebe Y, Yokota S, Matsumoto T, Hashimoto M, Sakai Y, et al. Low Carbohydrate diet (LCD) treated for three cases as diabetic diet therapy (2004) Kyoto Medical Association Journal 51: 125-129.
- Bando H, Ebe K, Muneta T, Bando M and Yonei Y. Clinical Effect of Low Carbohydrate Diet (LCD): Case Report (2017) Diabetes Case Rep 2: 124. https://doi.org/10.4172/2572-5629.1000124
- Muneta T, Kagaguchi E, Nagai Y, Matsumoto M, Ebe K, Watanabe H, et al. Ketone body elevation in placenta, umbilical cord, newborn and mother in normal delivery (2016) Glycat Stress Res 3: 133-140.
- Ebe K, Bando H, Muneta T, Bando M and Yonei Y. Remarkable improvement of glucose variability by Sodium–glucose cotransporter 2 (SGLT2) inhibitors using continuous glucose monitoring (CGM) (2019) Diabetes Case Rep 4:1. https://doi.org/10.15406/jdmdc.2020.07.00196
- Bando H. New era for useful add-on therapy (AOT) to diabetes by combined agents of insulin and glucagon-like peptide-1 receptor agonist (GLP-1RA) (2020) Int Med 2: 264-266. https://doi.org/10.5455/im.24928
- Jiang Y, Liu J, Chen X, Yang W, Jai W and Wu J. Efficacy and Safety of Glucagon-Like Peptide 1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus: A Network Meta-analysis (2021) Adv Ther 38: 1470-1482. https://doi.org/10.1007/s12325-021-01637-6
- Homepage of Xultophy®.
- Cohen ND, Audehm R, Pretorius E, Kaye J, Chapman LH and Colagiuri S. The rationale for combining GLP-1 receptor agonists with basal insulin (2013) Med J Aust 199: 246-249 https://doi.org/10.5694/mja12.11856
- Xultophy® 100/3.6 (insulin degludec and liraglutide injection): Novo Nordisk Medical
- Marso SP, Daniels GH, Frandsen KB, Kristensen P, Nauck AM, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes (2016) N Engl J Med 375: 311-322. https://doi.org/10.1056/nejmoa1603827
- 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2021 (2021) American Diabetes Association. 44: S111-S124. https://doi.org/10.2337/dc21-s009
- Taybani Z, Bótyik B, Katkó M, Gyimesi A and Várkonyi T. Simplifying complex insulin regimens while preserving good glycemic control in type 2 diabetes (2019) Diabetes Ther 10: 1869-1878. https://doi.org/10.1007/s13300-019-0673-8
- Price H, Bluher M, Prager R, Phan MT, Thorsted LB and Schultes B. Use and effectiveness of a fixed-ratio combination of insulin degludec/liraglutide (IDegLira) in a real-world population with type 2 diabetes: results from a European, multicentre, retrospective chart review study (2018) Diabetes Obes Metab 20: 954-962. https://doi.org/10.1111/dom.13182
- Novolin 30R Uses.
- Harris K and Nealy KL. The Clinical Use of a Fixed-Dose Combination of Insulin Degludec and Liraglutide (Xultophy 100/3.6) for the Treatment of Type 2 Diabetes (2018) Ann Pharmacother 52: 69-77. https://doi.org/10.1177/1060028017726348
- Yoo ER, Cholankeril G and Ahmed A. Treating Alcohol Use Disorder in Chronic Liver Disease (2020) Clin Liver Dis 15: 77-80.
- Kesavan K, Sureshkumar P. Impaired Glucose Regulation in Cirrhosis Liver-The Utility of Oral Glucose Tolerance Test (2019) J Med Sci Clin Res 7: 534-540. https://doi.org/10.18535/jmscr/v7i8.90
- Petersen M, Vatner D and Shulman G. Regulation of hepatic glucose metabolism in health and disease (2017) Nat Rev Endocrinol 13: 572-587. https://doi.org/10.1038/nrendo.2017.80
- Yu M, Benjamin MM, Srinivasan S, Morin EE, Shishatskaya EI, Schwendeman SP, et al. Battle of GLP-1 delivery technologies (2018) Adv Drug Deliv Rev 130: 113-130. https://doi.org/10.1016/j.addr.2018.07.009
- Lingvay I, Hansen T, Macura S, Marre M, Nauck MA, Woo V, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events (2020) BMJ Open Diabetes Res Care 8 :e001706. https://doi.org/10.1136/bmjdrc-2020-001706
- Capehorn MS, Catarig AM, Furberg JK, Janez A, Price HC, Tadayon S, et al. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10) (2020) Diabetes and Metabolism 46: 100-109. https://doi.org/10.1016/j.diabet.2019.101117
- Giorda CB, Nada E and Tartaglino B. Pharmacokinetics, safety, and efficacy of DPP-4 inhibitors and GLP-1 receptor agonists in patients with type 2 diabetes mellitus and renal or hepatic impairment. A systematic review of the literature (2014) Endocrine 46: 406-419. https://doi.org/10.1007/s12020-014-0179-0
- Ohki T, Isogawa A, Iwamoto M, Ohsugi M, Yoshida H, Toda N, et al. The effectiveness of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone (2012) Scientific World J. https://doi.org/10.1100/2012/496453
- Nouri-Vaskeh M, Khalili N, Khalaji A, Behnam P, Alizadeh L, Ebrahimi S, et al. Circulating glucagon-like peptide-1 level in patients with liver cirrhosis (2020) Archives of Physiology and Biochemistry 12: 1-6. https://doi.org/10.1080/13813455.2020.1828479
- Xultophy FDA Approval History: Drugs.com.
Corresponding author
Hiroshi
Bando, Tokushima University, Medical Research, Tokushima, Japan, Tel:
+81-90-3187-2485, E-mail: pianomed@bronze.ocn.ne.jp
Citation
Bando H, Sakamoto K, Ogawa T, Kondo N, Hatakeyama S, et al.. Clinical response to xultophy possibly varies from each different metabolic function (2021) Edelweiss Appli Sci Tech 5: 21-24.
Keywords
Insulin Degludec and Liraglutide
(IDegLira), glucagon-like peptide 1 (GLP-1), DUAL (Dual Action of Liraglutide
and Insulin Degludec), European Xultophy Treatment Retrospective Audit (EXTRA),
Japan LCD Promotion Association (JLCDPA).


 PDF
PDF