Introduction
Prostaglandins are essential mediator that are formed in many tissues and adjust many physiological functions, over normal and/ or patho physiological conditions [1-3]. They have many functions, as, the role of bone cells in establishing the hematopoietic stem cell, immunotherapy of cancer, female reproduction, platelet receptors, type I collagen structure, synthesis, and regulation nonsteroidal anti-inflammatory drugs for osteoarthritis [4] (Figure 1).
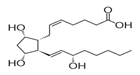
Figure 1: Dinoprostone (DIN) chemical structure.
Dinoprostone (DIN) in medicine identified as Prostaglandin E2, (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxyoct-1-enylcyclopentyl] hept-5-enoic acid is a naturally occurring prostaglandin used in medicine to induce labor and as an abortifacient [5,6]. Dinoprostone stimulates myometrial contractions in the gravid uterus that are similar to the contractions that occur in the term uterus during labor [7,8]. These contractions are usually sufficient to cause abortion [9].
Few analytical methods were developed and validated for determination of dinoprostone in drug substance, dosage form, human gastric mucosa, and in cultured tumor cells using HPLC with UV, laser induced fluorescence and electrospray ionization tandem mass spectrometric detectors. GC-MS was used also for determination of the drug in cultured tumor cells [10].
Due to the important biological role of prostaglandins, fast, simple and sensitive electrochemical method has to be developed for determination of DIN. Carbon Paste Electrodes (CPE) has been widely applied in the field of electrochemistry for the determination of low analyte concentrations due to their ease of fabrication, low cost and high sensitivity [11].
Modification of electrodes with various modifiers has been reported in recent years to improve sensitivity, selectivity, and detection limit [12-14]. Silica gel can be incorporated in paste with carbon and used as modifier. It has high adsorption capacity, insolubility in most solvents, and thermal stability, and high surface area of synthetic silica makes it valuable as support for various catalysts [15-20]. Gold Nanoparticles (GNPs) with large surface area, good biocompatibility, and high conductivity and electro catalytic activity have been used to increase sensitivity and improve detection limits [21-27].
The literature survey revealed that no attempt had been made to study the voltammetric behavior of dinoprostone. Therefore, the aim of the present work was to prepare a new sensor based on gold nanoparticles, silica, and graphite for rapid and selective electro analytical determination of DIN in drug substance and pharmaceutical product. Moreover the prepared electrode was characterized and the surface area was calculated.
Experimental
Materials and reagents
Dinoprostone was kindly supplied from Amriya Pharmaceutical Co., Egypt, and its purity was found to be 98.53% according to USP Pharmacopiea. Dinoglandin E2 (batch NO. 09477, Alexandria Co. for Pharmaceutical and Chemical Industeries) was labeled to contain 3 mg DIN per vaginal tablet. It was purchased from the local market. Silica gel was purchased from Sd. fine Chem. Ltd. Mumbai. Hydrogen tetrachloroaurate (HAuCl4) across organics New Jersey batch NO. AO321694 was purchased from Sigma-Aldrich. Britton-Robinson buffer (B-R buffer) was prepared by mixing different volumes of 0.04 M in H3PO4 (Adwic Co., Egypt), 0.04 M acetic acid (LOBA-Chemic Co., India), and 0.04 M boric acid (Polski EODZNN Chemiczne S.A. Co., Poland) with the appropriate amount of 0.2 M NaOH (Adwia Co., Egypt) to obtain the desired pH of 2.0-9.0. Buffer solutions were kept in a refrigerator [28]. All solutions were prepared from chemicals of analytical grade, and sterilized Milli-Q deionized water was used.
Standard solutions
Stock standard solution of dinoprostone (1 × 10-2 M) was prepared by dissolving appropriate amount of the drug in deionized water.
Preparation of electrodes
Carbon Paste Electrode (CPE) was prepared by mixing graphite powder (0.5 g) with paraffin oil (0.3 mL) in a glassy mortar. The carbon paste was packed into the hole of the electrode body and smoothed on a filter paper until its shiny appearance. Modified silica gel CPE (SMCPE) was prepared by mixing graphite powder with 5 % of its weight with silica gel. For better homogeneity, the resulting composite was dispersed in ethanol and stirred on a magnetic stirrer until the solvent completely evaporated, then about 3 mL of paraffin oil was added.
Gold silica modified electrode was prepared by immersing silica gel-modified CPE (SMCPE) composite into 6 mM hydrogen tetrachloroaurate (HAuCl4) solution containing 0.1 M KNO3 [29]. All the prepared electrodes were washed with double distilled water and dried carefully with a paper without touching the surface and then left to dry in air for 10 min before being used.
Instrumental and experimental setup
All
voltammetric measurements were performed using A Bio-logic SP 150 electrochemical
workstation. A One compartment cell and the three electrodes were connected to
the electrochemical workstation through a C3-stand. A platinum wire from BAS
(USA) was employed as the auxiliary electrode. The electrode potentials were
measured with respect to the reference electrode Ag/AgCl electrode from BAS
(USA). Sigma Plot 11 was used for the transformation of the initial signal. A
Cyberscan 500 digital (EUTECH Instruments, USA) pH meter with a glass
combination electrode served to carry out the pH measurement. Scanning Electron
Microscopy (
Electroanalytical measurements
Construction of calibration curve of dinoprostone; Aliquots equivalent to (0.1-2.0 mL), and (1-800 μL) from 1 × 10-3 M solutions of DIN were transferred into a series of 5-mL volumetric flasks for CPE and GNP/SMCPE respectively, using micropipette, and the volume was completed to the mark with B-R buffer pH 2. This solution was transferred to the electrolytic cell, and then Square Wave Voltammogram (SWV) was recorded. The peak current was measured at a scan rate of 10 mV s-1 using gold nanoparticles silica gel-modified CPE (GNP/SMCPE). Calibration curve was constructed by plotting the peak currents against drug concentrations.
Application to pharmaceutical product
Commercial pharmaceutical samples containing DIN was analyzed to evaluate the validity of the proposed method. Five vaginal tablets were finely mixed, and a weight equivalent to 15 mg of dinoprostone was dissolved in 30 mL of deionized water. Then 1.4 mL was transferred quantitatively to a 100 mL volumetric flask and completed to the mark with deionized water to obtain 10-3 M. Appropriate dilutions with deionized water were done to prepare samples in the quantification range.
Results and Discussion
Morphologies of different electrodes
The response of an electrochemical sensor was related to its physical morphology. The morphology of bare CPE (A), SMCPE (B), and GNP/SMCPE (C) were shown in Figure 2.

Figure 2: Scanning electron microscope images of (A) bare CPE, (B) SMCPE and (C) GNP/SMCPE.
The SEM image of CPE shows that its surface was characterized by a compact surface, isolated and irregularly shaped graphite, while the SEM image of GSMCPE shows that metallic nanoparticles are located at different elevations over the substrate. Moreover, a porous nanostructured film of gold nanoparticles was noticed which extremely enhanced the active surface area of GNP/SMCPE and might be very important to promote electron transfer.
Electrochemistry of dinoprostone
Preliminary investigation using cyclic voltammetry shows a behavior of irreversible oxidation of DIN at bare CPE, SMCPE, and GNP/SMCPE. Figure 3 shows typical cyclic voltammograms of 1.0 × 10-2 mol L-1 of DIN, in B-R buffer pH 2.0, at a scan rate of 100 mV s-1, recorded at three electrodes under investigation. At bare CPE, the oxidation peak current was observed to be 30.4 μA, while in the case of SMCPE, the oxidation peak current was found to be 40.1 μA and the best one is GNP/SMCPE, which has a value of 80 μA.
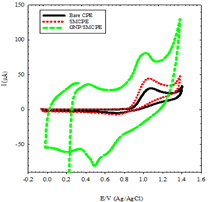
The potential of different electrodes were found in order of, bare CPE, SMCPE, and GNP/SMCPE respectively (1.13 V compared to 1.12 V, and 1.16 V), due to the improvement in the reversibility of the electron transfer process and a larger real surface area of the modified electrode. The electro deposition of gold nanoparticles on GNP /SMCPE resulted in an observable increase in the peak current, which indicated an improvement in the electrode kinetics and increase in the potential of oxidation substantial, where GNP/SMCPE acts as a cation exchange [29,30] that attracts the positively charged DIN.
Selection of the Optimum Experimental Conditions
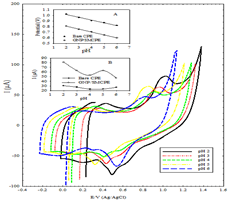
Effect of pH: In order to ascertain that electrocatalytic oxidation of DIN would be pH dependent, the voltammetric response of DIN was investigated in solutions with varying pH from 2.0 to 6.0 in order to optimize the electrocatalytic response. Figure 4 shows the cyclic voltammograms of the oxidation peak currents of DIN at different pH values using B-R buffer at bare CPE, and GNP/SMCPE electrode. Higher anodic current for DIN at pH 2 is due to major microspecies at this pH.
Moreover, DIN oxidation is a one-electron process, which may be attributed to the oxidation of double bonds [31-33]. DIN carries positive charge that can be attracted by the negative charge of the electrode; the suggested electrochemical oxidation of DIN was depicted in Scheme 1.
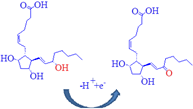
Scheme 1: Suggested oxidation mechanism of dinoprostone.
A comparison between the anodic peak current at different pH values of bare CPE, and GNP/SMCPE show that GNP/SMCPE displays higher anodic current for DIN than bare CPE which indicates the effect of gold on the catalytic oxidation processes as shown in Figure 2. It is observed that as the pH values increase, the peak potential shifts toward less positive values, which indicates the participation of protons in the electrode process and that the electrocatalytic oxidation of DIN is a pH-dependent reaction. The relationship between the anodic peak potential and the solution pH value at bare CPE and GNP/SMCPE could be fit to the linear regression equation of Epa (V)=1.1132-0.0493 pH, with a correlation coefficient of r=0.9973 and Epa (V)=0.9118-0.0521 pH, with a correlation coefficient of r = 0.9994 respectively. The slope was found to be 49.3 mV/pH and 52.1 mV/pH units at bare CPE and GNP/SMCPE respectively over the pH range from 2 to 6, which is close to the theoretical value of -48.3 mV. This indicated that the number of protons and transferred electrons involved in the oxidation mechanism are equal [34].
Effect of scan rate: The interfacial reaction of the drug at each electrode was identified by recording the cyclic voltammograms of 1 × 10-3 M solution at different scan rates (ν) 10-250 mV s-1 in B-R buffer (pH 2.0). Typical CV curves of DIN at different scan rates were shown in Figure 5.
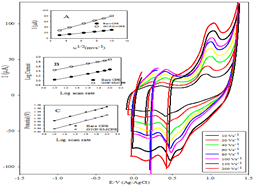
Figure 5: Cyclic voltammograms of 1.0 × 10-3 mol L-1 DIN at GNP/SMCPE in 0.04 M B-R buffer pH 2 from 10 to 200 mV s-1, Inset A: plot of Ip vs. v1/2. Inset B: plot of log Ip vs. log v. Inset C: plot of Ep vs. log v.
Figure 5 inset A showed that the peak current increased linearly with increasing the square root of scan rate up to a scan rate of 100 mV s-1, according to regression equation [35]:
ip= (2.69 x 105) n 3/2 A Co x Do ½ ν1/2
In this equation, ip is the peak current density (μA cm-2), n is the number of electrons appearing in half-reaction for the redox couple, ν is the scan rate at which the potential is swept (V s-1), C is the analyte concentration, A is the electrode area (0.071 and 0.118 cm2 for bare CPE, and GNP/SMCPE respectively), and Do is the electroactive species diffusion coefficient (cm2 s-1). The apparent diffusion coefficient, Dapp, of DIN in B-R buffer (pH 2) was calculated from Cyclic Voltammetry (CV) experiments which increases from 8.9 × 10-7 cm2 s-1 in case of using bare CPE to 4.3 × 10-5 cm2 s-1 after the functionalization of bare CPE surface with gold nanoparticles. This indicated the quick mass transfer of the analyte molecules toward GNP/SMCPE surface from bulk solutions and fast electron transfer process of electrochemical oxidation of the analyte molecule at the electrode-solution interface. The calculated Dapp values also showed that gold improves the electron transfer kinetics at the electrode/solution interface, suggesting that the reaction is a diffusion-controlled electrode reaction.
Direct proportionality was obtained between log current and log scan rate in range of 10-100 mV s-1 (Figure 5 inset B), giving the following equation:
log I=0.6003+0.435 log ν r=0.9994 for Bare CPE
log I=1.0134+0.444 log ν r=0.9992 for GNP/SMCPE
The value of the slope of the obtained linear relations is less 0.5 which implies that the electroactive species are transported by a diffusion process with an adsorption contribution [36]. From the different investigated scan rates, the 100 mVs-1 gave the best voltammograms and higher selectivity.
The electrochemical oxidation peak potential (Ep) was also dependent on the scan rate, where increasing the scan rate resulted in a shift to more positive potentials, as shown in Figure 5 inset C.
Ep(V)= 0.9046+0.0823 log ν(Vs-1) r=0.9993
Ep(V)=0.8562+0.0819 log ν(Vs-1) r=0.9996
In order to determine the kinetic parameters of the electron-transfer process for the DID oxidation on the GNP/SMCPE, Lavirons theory [37,38] for irreversible processes was applied to calculate the number of electron transferred.
E=Eo+2.303 RT/αnF[log RTKo/αnF]+2.303RT/αn F(log ν)
Where, R is the gas constant (8.314 J K mol-1), T is the temperature (298 K), F is the faraday constant (96485 Cmol-1), α is the electron transfer coefficient, and n is the number of the electrons and αn can be calculated from the slope of potential against log scan rate. In this system, the slope were 0.0823 and 0.0819, αn was calculated to be 0.719 and 0.720 for bare CPE and modified electrode respectively, since for a totally irreversible electron transfer, α assumed as 0.5, then n was calculated to be 1.4 which indicated that one electron were involved in the oxidation of DIN.
Method validation
Linearity, LOD, and LOQ: Under the above optimum conditions, the linearity using SWV was carried out where good correlation between the oxidation peaks current (I) and concentration was found in ranges of 2x10-5-4 x10-4 mol L-1, and 2x10-7-1.6x10-4 mol L-1 using bare CPE, and GNP/SMCPE (Figure 6). The Limits of Detection (LOD) and the Limits of Quantitation (LOQ) were calculated from the oxidation peak currents of the linear ranges according to ICH guideline [39]. LOD and LOQ values confirmed the sensitivity of GNP/SMCPE over bare CPE. The calibration equation parameters and necessary validation data are shown in Table 1.
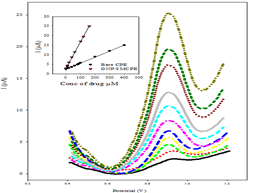
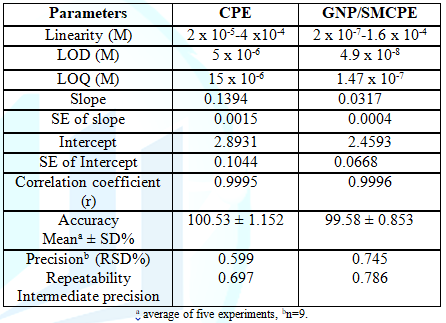
Accuracy: The accuracy of the proposed method for determination of DIN in drug substance was shown in Table 1. The mean percentage recoveries were evaluated and satisfactory results were obtained. The accuracy was further assessed by application of standard addition technique (Table 2).
Precision: The intraday and interday precision were assessed by analyzing three concentration levels in triplicate in a single assay run, and on three separate assay runs for the drug, RSD% were less than 2%. This level of precision was adequate for the quality control analysis of the drug as shown in Table 1.
Robustness: The robustness of the proposed method was demonstrated by constancy of the peak current with deliberated minor changes in the experimental parameters. The studied variables included; the change in pH (2.0 ± 0.2). These minor changes that may take place during the experimental operation did not affect the peak current intensity of the studied drug, indicating the reliability of the proposed method during normal usage.
Application of the proposed SWV method for the determination of dinoprostone in pharmaceutical preparation
The proposed SWV method was successfully applied to determine DIN in its pharmaceutical formulation. The obtained results are listed in Table 2. The specificity of the proposed SWV voltammetric method was proven by its ability to determine DIN in pharmaceutical formulation without interference from excipients that commonly present.
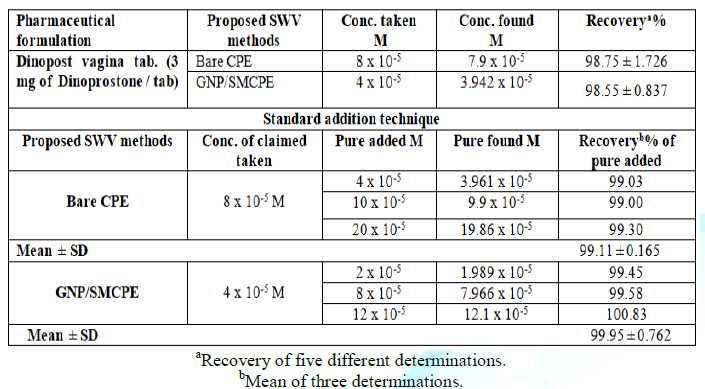
In addition, the validity of the proposed SWV method was assessed by the standard addition technique, Table 2.
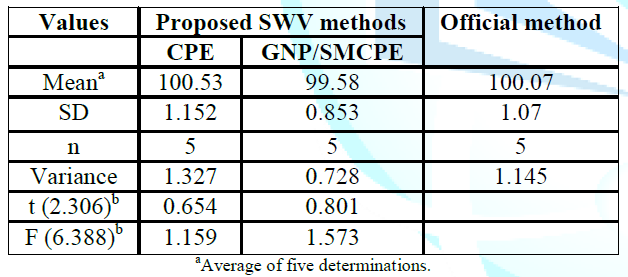
Statistical comparison between the results obtained by the proposed method and the official method using student t-test and F ratio revealed no significant differences with respect to accuracy and precision at probability 0.05% and the data was presented in Table 3.
Conclusion
Inexpensive and eco-friendly SWV method was developed and validated for rapid sensitive determination of DIN in drug substance and pharmaceutical dosage form. The literature review revealed no attempted had been made for electrochemical determination of DIN. The proposed method was based on the electrochemical oxidation of DIN at both bare CPE and gold nanoparticle/silica-modified CPE as a new fabricated sensor which causes an enhancement in the anodic peak current. The results indicate the validity of the methods for application in routine quality control, since it is characterized by high reproducibility and selectivity.
References
1. Phillis JW, Horrocks LA and Farooqui AA. Cycloxygenases, lipoxygenases, and epoxygenases in CNS: Their role and involvement in neurological disorders (2006) Brain Res Rev 52: 201-243. https://doi.org/10.1016/j.brainresrev.2006.02.002
2. Bazan NG, Colangelo V and Lukiw WJ. Prostaglandins and other lipid mediators in Alzheimers disease (2002) Prostaglandins Other Lipid Mediat 68-69: 197-210. https://doi.org/10.1016/S0090-6980(02)00031-X
3. Chen C and Bazan NG. Lipid signaling: sleep, synaptic plasticity, and neuroprotection (2005) Prostaglandins Other Lipid Mediat 77: 65-76. https://doi.org/10.1016/j.prostaglandins.2005.07.001
4. Backlund MG, Mann JR and Dubois RN. Mechanisms for the prevention of gastrointestinal cancer: the role of prostaglandin E2 (2005) Oncology 69: 28-32. https://doi.org/10.1159/000086629
5. United States Pharmacopoeia (USP 40) (2017) United States Pharmacopoeia Convection, Inc., Rockville, USA
6. Pernoll ML and Benson RC. Current obstetric and gynecologic diagnosis and management (6th edn) (1987) CT: Appleton and Lange, Norwalk, USA 608.
7. Reynolds JEF. Martindale, the extra pharmacopeia (29th edn) (2011) London: The Pharmaceutical Press, UK 1367.
8. Christiansen NJ, Bygdeman M, and Green K. Comparison of different prostaglandin analogues and laminaria for pre-operative dilatation of the cervix in the late first trimester abortion (1983) Contraception 27: 56-61.
9. Lange AP. Primary pulmonary hypertension during pregnancy: A case report(1983) Acta Obstet Gynecol Scand Supp 113: 117-124.
10. Yang P, Felix E, Madden T, Fischer SM and Newman AR. Quantitative high-performance liquid chromatography/electrospray ionization tandem mass spectrometric analysis of 2- and 3-series prostaglandins in cultured tumor cells (2002) Anal Biochem 308: 168-177. https://doi.org/10.1016/S0003-2697(02)00218-X
11. Mohamed MA, Atty SA, Salama NN and Banks CE. Highly Selective Sensing Platform Utilizing Graphene Oxide and Multiwalled Carbon Nanotubes for the Sensitive Determination of Tramadol in the Presence of Co‐Formulated Drugs (2017) Electroanalysis 29: 1038-1048. https://doi.org/10.1002/elan.201600668
12. Dryhurst G and McAllister DL. Carbon electrodes, in laboratory techniques in electroanalytical chemistry, Dryhurst G, McAllister DL, Kissinger P and Heineman WR(Eds) (1984) Marcel Dekker Inc., USA.
13. Wang J. Analytical electrochemistry (2nd edn) (2000) Wiley-VCH, USA. https://doi.org/10.1002/0471228230
14. Mohamed MA, Atty SA, Merey HA, Fattah TA, Foster CW, et al. Titanium nanoparticles (TiO2)/graphene oxide nanosheets (GO): an electrochemical sensing platform for the sensitive and simultaneous determination of benzocaine in the presence of antipyrine (2017) Analyst 142: 3674-3679.
15. Yang RT. Adsorbent: fundamentals and application (1999) John Wiley, USA 131-134. https://doi.org/10.1002/047144409X
16. Keller R. The Chemistry of Silica (1979) John Wiley, USA 896.
17. Yantasee W, Lin Y, Zemanian TS and Fryxell GE. Voltammetric detection of lead(II) and mercury(II) using a carbon paste electrode modified with thiol self-assembled monolayer on mesoporous silica (SAMMS) (2003) Analyst 128: 467-472.
18. Sayen S, Gerardin C, Rodehuser L and Walcarius A. Electrochemical Detection of Copper (II) at an Electrode Modified by a Carnosine-Silica Hybrid Material (2003) Electroanalysis 15: 422-430. https://doi.org/10.1002/elan.200390049
19. Yantasee W, Lin Y, Fryxell GE and Busche BJ. Environmental Applications of Nanomaterials: Synthesis, Sorbents and Sensors (2003) Anal Chim Acta 502: 207-212.
20. Marino G, Bergamini MF, Teixeira MFS and Cavalheiro Talanta ETG. Evaluation of carbon paste electrode modified with organo-functionalized amorphous silica in for the determination of cadmium(II) by using differential pulse anodic stripping analysis (2003). 59: 1021-1028.
21. Huang W, Qian W, Jain PK and El-Sayed MA. The effect of plasmon field on the coherent lattice phonon oscillation in electron-beam fabricated gold nanoparticle pairs (2007) Nano Lett 7: 3227-3234. https://doi.org/10.1021/nl071813p
22. Khatri OP, Murase K and Sugimura H. Structural organization of gold nanoparticles onto the ITO surface and its optical properties as a function of ensemble size (2008) Langmuir 24: 3787-3793.
23. De Oliveira MK, Thaís dos Santos CC, Dinelli RL, Marinho ZJ, Lima CR, et al. Aggregates of gold nanoparticles with complexes containing ruthenium as modifiers in carbon paste electrodes (2013) Polyhedron 50: 410-417.
24. Li F, Song JX, Gao DM, Zhang QX, Han DX, et al. Simple and rapid voltammetric determination of morphine at electrochemically pretreated glassy carbon electrodes (2009) Talanta, 79: 845-850.
https://doi.org/10.1016/j.talanta.2009.05.011
25. Atta NF, Galal A, Abu-Attia FM and Azab SM. Simultaneous determination of paracetamol and neurotransmitters in biological fluids using a carbon paste sensor modified with gold nanoparticles (2011) J Material Chem 21: 13015-13024.
26. Atta NF, Galal A and Azab SM. Novel sensor based on carbon paste/nation modified with gold nanoparticles for the determination of glutathione (2012) Anal Bioanal Chem 404: 1661-1672.
27. Atta NF, Galal A and Azab SM. Determination of morphine at gold nanoparticles/Nafion® carbon paste modified sensor electrode Analyst (2011) 136: 4682-4681. https://pubs.rsc.org/en/content/articlelanding/2011/an/c1an15423k#!divAbstract
28. Joseph E Reynolds III, Josowicz M, Tyler P, Russell BV and Kyril MS. Spectral and redox properties of the GFP synthetic chromophores as a function of pH in buffered media (2013) Chem Commun 49: 7788-7790.
29. Atta NF, Galal A, Abu-Attia FM and Azab SM. Carbon Paste Gold Nanoparticles Sensor for the Selective Determination of Dopamine in Buffered Solutions (2010) J Elechtrochem Soc 157: F116-F123.
30. Laviron E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems (1979) J Electroanal Chem 101: 19-28. https://doi.org/10.1016/S0022-0728(79)80075-3
31. Smith RJL and Masheder D. Amine oxidation. Part IX. The electrochemical oxidation of some tertiary amines: the effect of structure on reactivity (1976) J Chem Soc Perkin Trans 2 47-51.
32. Siddappa K, Mallikarjun M, Reddy T and Tambe M. Simple and Sensitive Extractive Spectrophotometeric Method for the Assay of Mebeverine Hydrochloride in Pure and Pharmaceutical Formulations (2008) J Chin Chem Soc 55: 1062-1068. https://doi.org/10.1002/jccs.200800155
33. Hoh GLK, Barlow DO, Chadwick AF, Lake DB and Sheeran SR. Hydrogen peroxide oxidation of tertiary amines (1963) J Am Oil Chem Soc 40: 268-271. https://doi.org/10.1007/BF02633687
34. Majidi MR, Jouybanb A and Zeynali KA. Voltammetric behavior and determination of isoniazid in pharmaceuticals by using overoxidized polypyrrole glassy carbon modified electrode (2006) J Electroanal Chem 589: 32-37. https://doi.org/10.1016/j.jelechem.2006.01.016
35. Atta NF, Darwish SA, Khalil SE, and Galal A. Effect of surfactants on the voltammetric response and determination of an antihypertensive drug (2007) Talanta 72: 1438-1445. https://doi.org/10.1016/j.talanta.2007.01.053
36. Gosser DK. Cyclic voltammetry: simulation and analysis of reaction mechanism (1993) Wiley-VCH, USA 156.
37. Gushikem Y and Rosatto SS. Metal Oxide Thin Films Grafted on Silica Gel Surfaces: Recent Advances on the Analytical Application of these Materials (2001) J Braz Chem Soc 12: 695-705.
38. Bard AJ and Faulkner LR. Electrochemical methods: fundamentals and applications (2001) Wiley, USA 864.
39. Validation of analytical procedures: text and methodology Q2 (R1) (2005) International conference on harmonization (ICH) harmonized tripartite guideline, Switzerland 1-13.
*Corresponding author
Maha A Elabd, Department of Pharmaceutical Chemistry, National Organization for Drug Control and Research, 6 Abou-Hazem st., Giza, Egypt, E-mail: mahaelabd@hotmail.com
Citation
Atty SA, Walash M, Toubar S, AbouEl-Alamin MM, Elabd EA, et al. Spot on gold nanoparticles/silica modified electrode for rapid sensitive determination of dinoprostone (2019) Edelweiss Chem Sci J 2: 17-22
Keywords
Dinoprostone, Square wave voltammetry, Sensor, Carbon paste electrode, Gold nanoparticles/silica.


 PDF
PDF