Introduction
Our planet is in trouble because of global warming,
the heating of earth’s surface, oceans and atmosphere. Glaciers are melting,
sea levels are rising, and wild fires and heat waves are getting more severe. Carbon dioxide is
one of the major greenhouse gases. To avoid global warming, a significant shift
from "low carbon"
to "decarbonization"
society is necessary. From this standpoint, we have published chemical
synthesis of fuel hydrocarbon from CO2 and activated water [1]. In
fossil materials such as all mineral oil or the products, the 14C
contents could decay over millions of years, therefore no 14C can be
detected anymore [2]. On the other hand, in fixation of atmospheric CO2,
the synthesized
organic materials should contain 14C. In the
chemical synthesis of oil, origin of CO2 was thought to be
atmospheric air, but scientific evidence was not proved. CO2 in the
air is composed of about 99% 12C, about 1% 13C, and about
one trillionth radioisotope 14C. Firstly, we tried to use 13C-CO2.
However, noise of background was not negligible. Thereafter, we decided to
measure beta-decay of 14C incorporated in newly synthesized
hydrocarbon.
Materials and Methods
According to the previously mentioned method (1),
new oil was synthesized as follows. In this process, oxygen gas is converted to
ozone, and further to reactive oxygen species such as superoxide anion radicals
and hydroxyl radicals. The reactive oxygen species may reduce carbon dioxide to
carbon monoxide, as follows,
2CO2
⇒ 2CO +O2 reaction 1
By using TiO2 photocatalyst, H2O
was decomposed into H2 and O2 as follows,
2H2O
⇒ 2H2+O2 reaction 2
As a total,
CO2+H2O
⇒ CO+H2+O2 reaction
3
The oil generation reaction may occur as radical
polymerization in emulsion and be written as follows,
nCO+(2n+1)H2
⇒ CnH2n+2+nH2O
reaction
4
At each amplification cycle, volume and weight of
oil and activated water were measured before and after the cycle. Then real
increase of oil was recorded. Oil should be added in this reaction, because it
is a template synthesis [1]. Analytical data and characteristics of newly
generated oil and original oil have been compared and reported in this journal
[1]. Concentration of
the atmospheric CO2 is 407 ppm which was reported by world
meteorological organization in 2019 (https://gaw.kishou.go.jp/) [3].
After each cycle of oil amplification, 10 ml sample
was mixed with 10 ml of scintillation cocktail (Ultima Gold F, PerkinElmer,
USA). Hitachi ALOKA Accuflex LSC-7200 was used for scintillation counting. Measurement
time was 300 min. Since hydrocarbon is week quencher, the newly synthesized oil
was measured as soon as possible for liquid scintillation counting. Carbon
content of oil was measured by using MICRO CORDER JM11 (J-SCIENCE LAB CO) and
Flash Smart Organic Elemental
Analyzer (Thermo Fisher Scientific CO). Weighing balance
used was MSA 2.7S-000-DM (SARTORIUS JAPAN CO).
Results and Discussion
Chemical
synthesis of hydrocarbon
Commercial light oil was purchased from gasoline
station and used for first chemical synthesis of hydrocarbon. The mixture of
original light oil and newly synthesized oil is called as Amplification Cycle 1
(AC1). AC1 sample was used for the second synthesis. Likewise, the chemical
synthesis was repeated ten times (from AC1 to AC10). Volume and weight of oil
and activated water were carefully measured before and after each cycle. The
increased % of oil (indicated as red circle) was accumulated ten times (Figure 1). The total increase of AC10
was around 46.2 %.
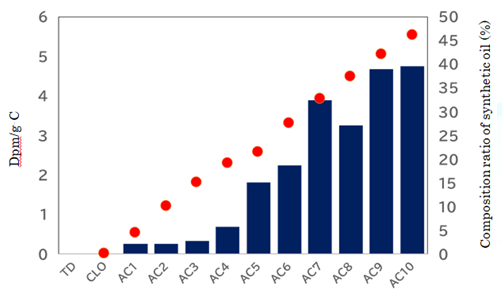
Measurement of 14C in synthesized hydrocarbon
Since the amount of 14C in the
atmospheric CO2 is about one trillionth, the extremely low
concentration of radioisotope 14C has to be measured with Liquid
Scintillation Counting (LSC) [3]. The direct measurement of an
organic sample in the LSC is always advantageous if a sample such as newly
synthesized oil can be dissolved in the scintillation cocktail such as Ultima
Gold F (PerkinElmer, USA). The organic sample should also show no or only
little color. We adopted extremely long counting times (300 min). The
incorporation of 14C into new oil increased as amplification cycle
increased (Figure 1). About 4.6 dpm/gC was determined at 46.2% increase of oil,
where carbon content of oil was measured as 86.04%. As the volume of original
fossil oil was increased up to 1.462 times, real value of true incorporated radioisotope in
synthesized oil is 4.6 × 1.462 ÷ 0.462=14.6 dpm/gC. When 14C content
in ethanol of Japanese wine was measured, the average content was 15.1 dpm/gC
[4]. It was also reported that 14C content in ethanol of various
liqueurs including brandy, whisky, vodka etc. ranged from 16 to18 dpm/gC [5]. Therefore,
14.6 dpm/gC of oil is nearly the same level with biogenic material. These data
clearly shows that atmospheric CO2 was fixed to form new oil. It is
sure that a series of these experiments contribute to carbon neutrality.
Conclusion
The 14C content of newly synthesized
hydrocarbon was measured by liquid scintillation counting. These data clearly
showed that atmospheric CO2 was directly fixed into hydrocarbon.
This process must contribute to decarbonization society in the future.
References
- Imanaka T and Takemoto T. chemical synthesis of fuel
hydrocarbon from co2 and activated water, and purification of
commercial light oil for dream oil (2019) Edelweiss Chem. Sci J 2: 23-26. https://doi.org/10.33805/2641-7383.111
- Edler R and Kaihola L. Determination of the 14C
content in fuels containing bioethanol and other biogenic materials with liquid
scintillation counting (2007) LSC Application Note 43, PerkinElmer, United
States.
- World
Meteorological Organization (Global Atmosphere Watch).
- Fuma S, Inoue Y, Sato N and Hirano M. Report
National Institue of Radiological Sciences (1998) Annual Report 6-9, Japan.
- Saito M, Nakamura M and Yamazaki M. Report Tokyo
Metropolitan Industrial Technology Research Institute (2009) Annual Report 16-19,
Japan
Corresponding author
Tadayuki Imanaka, The Research
Organization of Science and Technology, Techno-Complex Rm244, Ritsumeikan
University, Noji-Higashi, Kusatsu, Shiga 525-8577, Japan, E-mail: imanaka@sk.ritsumei.ac.jp
Citation
Imanaka T, Takemoto T and Imanaka H. Direct
fixation of atmospheric co2 towards chemical synthesis of fuel
hydrocarbon (2021) Edelweiss Chem Sci J 4: 1-2.
Keywords
CO2 fixation, Measurement of 14C,
Beta-decay of 14C, Fuel hydrocarbon


 PDF
PDF