Introduction
Very little research has actually been focused on the short chain fatty acid SCFA production of one of mankind’s most prevalent pathogens, Streptococcus mutans. S. mutans is a key dental pathogen, long associated with one of the most common diseases of humankind [1]. The incidence of dental caries in over 98% of the 65 years and above population demonstrates the universality of this disease [2]. However, dental caries is totally preventable, being the result of a dysbiosis of the oral cavity, with both the increased presence of oral pathogens and the decreased level of protective commensals, particularly the nitrate reducing commensals [3]. The oral microbiome shifts significantly over the different time periods of child development and in response to the diet [4]. Unfortunately, the oral microbiome has had the same response as the Gut microbiome to the massive dietary shifts; the Agricultural, Industrial, and more currently, the Fast Food revolutions [5]. That is, there has been a relative decrease in diversity coinciding with an increase not only in the number of pathogens, but also their pathogenicity [6]. Efforts to reduce the levels of S. mutans in infants and children with xylitol and preventing dental caries have been successful [7,8]. However, other bacterial and fungal organisms have now been closely identified with the development of dental caries [9]. Scardovia wiggsiae is a Bacillus bacteria found extensively associated with Severe-Early Childhood Caries (S-ECC) [10]. Scardovia wiggsiae and Slackia exigua have been reported to be involved in the early caries development [11]. Candida albicans, a fungal organism, helps with the biofilm production by increasing the extracellular polysaccharide matrix which protects S. mutans from anti-microbials and commensals such as Streptococcus oralis [12]. Lactobacilli inhibit the colonization of Candida albicans, hence decreasing the polysaccharide matrix, exposing the S. mutans to the bactericins or hydrogen peroxide of its natural competitors, other Streptococcus species [13]. In addition, Streptococcus oralis produces hydrogen peroxide that inhibits the anaerobic Streptococcus mutans growth [14,15]. Indeed, Probiora© probiotic, a commercially available probiotic product, contains Streptococcus oralis, uberis and rattus, and claims to inhibit several key dental pathogens [16-19]. Probiotics have been reported to be an important adjunct in preventive dental care [20-22]. Xylitol has been studied for its effect on the lactobacillus bacteria, a genus that consists of many probiotics, and it has been reported that xylitol does not significantly inhibit the Lactobacilli.
Polyols,
sugar alcohols, have a distinct effect upon the microbiome and have long been
utilized in oral medicine to reduce pathogen populations and also are referred
to as prebiotics. Significant research studies have long demonstrated the
effectiveness of polyol ingestion for the prevention of dental caries and now
also for periodontal pathology [23,24]. A significant portion of the
effectiveness is reportedly due to the polyol effect on the pathogenic
microbiome [25]. Pathogens are more susceptible to the inhibitory effect of
xylitol than the commensal bacteria. Studies of xylitol demonstrated little
effect on probiotic bacteria, and long clinical studies demonstrate the biofilm
effects are long term, and even are transmissible from mother to child [26,27].
Polyols safely inhibit the growth and biofilm production of oral pathogens that
also have a significant effect systemically, such as, S. mutans causing hemorrhagic stroke [28]. In addition, polyols
shift the metabolites (acetate, lactate and propionate) produced by the oral
microbiome [29]. Carious dentin contains both acetate and propionate, produced
by cariogenic bacteria prompting the research into the propionic acid
production by S. mutans [30]. Polyols
have been reported to shift the production of the organic acids of the oral
microbiome in the young patient population creating a long term benefit [31].
Materials and Methods
BHI
broth supplemented with 2% or 10% sucrose containing no polyols or either
erythritol or xylitol at various concentrations was used for this study. S. mutans (ATCC 35668) was grown
aerobically. After 48 hours of growth the supernatant were harvested and
centrifuged to pellet bacteria. Supernatants were removed from bacterial
pellets, filtered through 0.22 micron filters and stored in sterile cryovials
until submitted for SCFA analysis at the IMSERC Mass Spectrometry Center
(Northwestern University).
The
instrument utilized was an Agilent Technologies (Santa Clara, CA 95051) system
configured from three components, a 5973 mass selective detector, a 6890N gas
chromatographer, and a 7697A headspace sampler. Mixture components separation
was achieved by using a FFAP column (Agilent J&W DB-FFAP; is a
nitroterephthalic-acid-modified PEG) and a 10 minute temperature gradient
(initial temperature at 50 °C, hold for 1 minute, and ramp to 240 °C in 6
minutes, and held for 3 minutes, to give a total run time of 10 minutes). The
standards of each of the SCFA samples were made in water and linearity
established before test samples were committed to analysis. The linearity of
the test samples were also demonstrated before the data was accepted. The SCFA
test samples were analyzed as submitted without need for any further
processing. Headspace oven incubation times of 15 minutes were used for both
test samples and standard solutions.
Results
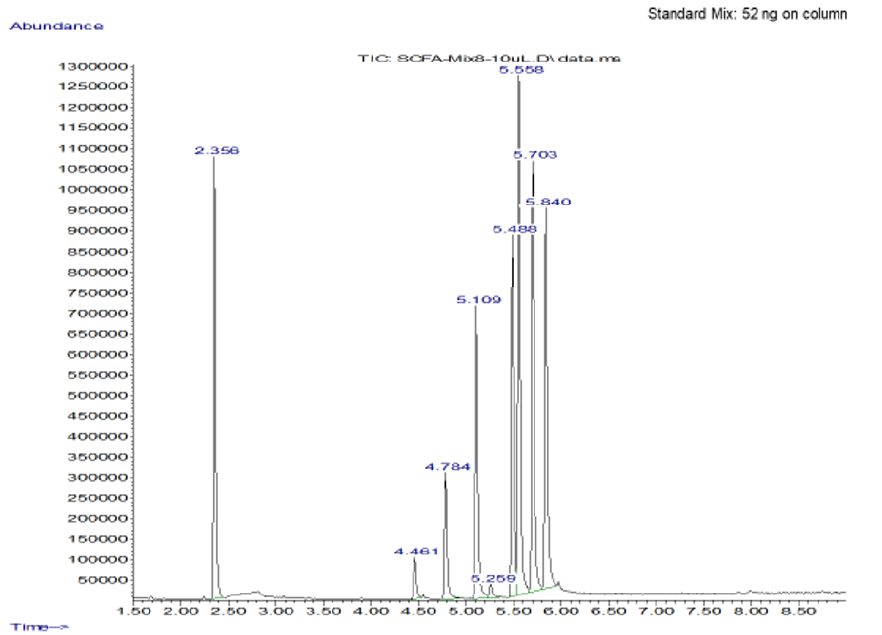
Representative
data
Standard Positive Control Negative Control
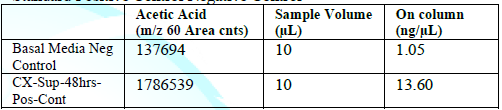
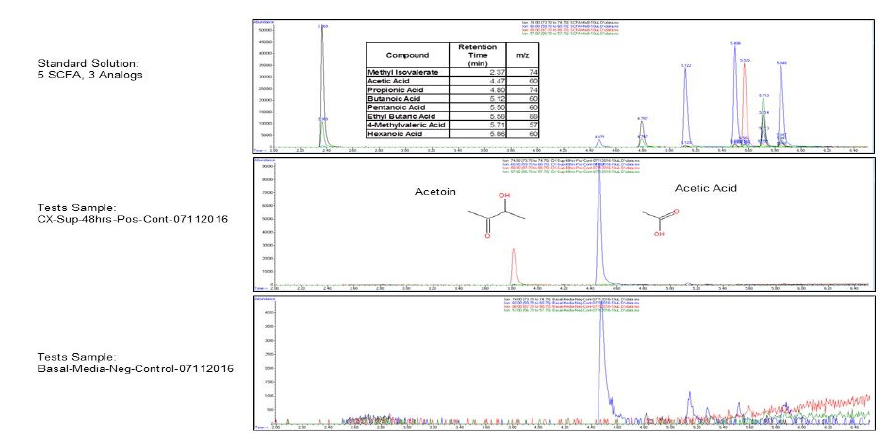
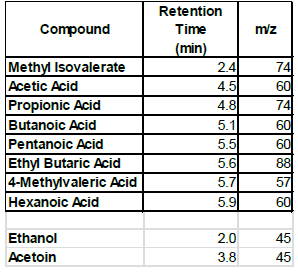
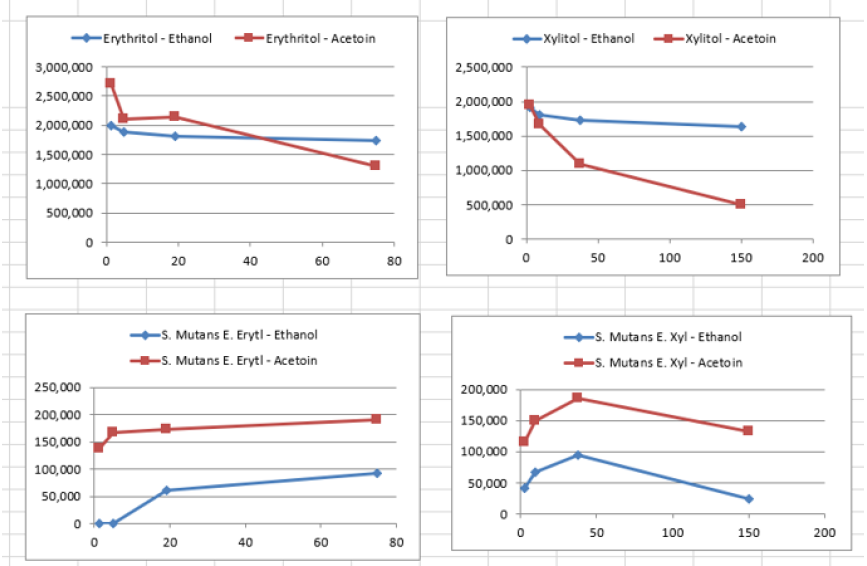
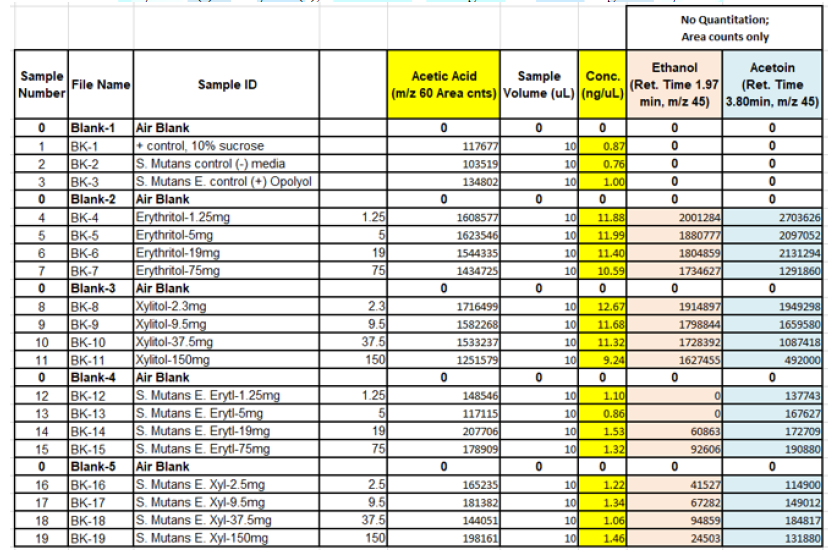
When the BHI broth was supplemented with 2% or 10% sucrose but containing no polyols was used to grow S. mutans, the following short chain fatty acids were produced: methyl isovalerate, acetic acid, propionic acid, butanoic acid, pentanoic acid, ethyl butaric acid, 4-methylvaleric acid, hexanoic acid. Note that this particular strain of S. mutans did not produce lactic acid. When the BHI broth supplemented with 2% or 10% sucrose containing erythritol was used as media for this S. mutans strain, the following were produced: ethanol, acetoin, and acetic acid. Note that propionic acid was not detected.
Discussion
Modification
of the microbiome metabolites with polyols, or possibly the diet in general,
has greater effects than previously appreciated. Research into epigenetic
effects, the response of the genome to environmental factors, including the
influence of SCFAs has greatly increased. Therefore, significant study of the
microbiome, the microbiome effect epigenetically, and the modification of the
microbiome via polyols, deserves intense interest. In this pilot study, we analyzed
the effects of polyols on only one pathogen, but the effect was demonstrative.
In
humans, the gut microbiota plays an important role in many functions, such as
modulation of the immune system, production of vitamins and amino acids, the
detoxification of harmful chemicals, and the breakdown of dietary fiber into
short chain fatty acids. In this study, we examined the role that S. mutans may play in the production of
short chain fatty acids in vitro, and how the changing environment (media with
polyol added) has an impact on what types of SCFAs are produced. When a strain
of S. mutans was grown with sucrose,
it produced different SCFAs than when grown with the polyol erythritol. Most
notably, when grown with erythritol, this strain no longer produced propionic
acid.
By
shifting production away from propionic acid, the erythritol environment allows
other SCFAs to dominate amongst the metabolites of S. mutans. Propionic infusions into adult rat cerebral ventricles
produces behaviors associated with Autistic Spectrum Disorder (A.S.D.) [32] and
produces reversible repetitive dystonic behaviors, hyperactivity, turning
behavior, retropulsion, caudate spiking, and the progressive development of
limbic kindled seizures, coupled with neuroinflammatory, metabolic and
epigenetic changes suggesting that it has central effects [33,34]. MacFabe, et
al. also administered propionic acid subcutaneously and intra peritoneal
finding very similar results [35,36]. Exposure of human lymphoblastoid cell
lines to propionic acid elicited an atypical immunologic response [37,38]. On
the other hand, propionic acid also has positive health effects with adults,
such as anti-obesity, anti-inflammatory, and cholesterol lowering effects [39].
Calcium propionate has been utilized as a food preservative although the use
appears to be decreasing. A large fast food restaurant chain recently announced
discontinuing calcium propionate due to concerns over behavioral changes in
children consuming calcium propionate preserved bread [40].
Additional
laboratory study is required to test other species besides S. mutans, specifically the propionic producing Clostridium
histolyticum and bolteae. By adding polyols to the diet, we could
potentially shift the SCFA production to decrease the amount of propionic acid
produced. Various low refined carbohydrate diets may help with ASD by reducing
the substrates needed for SCFA production, and the supplementation of foods
high in complex fibers may exert a therapeutic response in children by
preferentially increasing the production of another SCFA, butyrate, over the
production of propionic acid [41].
Short-Chain
Fatty Acids (SCFA) formed by microbial fermentation have an important effect on
colonic health [42,43]. Butyrate particularly has an important role in the
metabolism and normal development of colonic epithelial cells and has been
demonstrated to be protective against cancer and ulcerative colitis [44].
Butyrate is considered to be a preferred energy source for colonic epithelial
cells and plays an important role in maintaining colonic health in humans. In a
study of the colonic bacteria by Barcenilla et al, fifty percent of the
butyrate-producing isolates were net acetate consumers during growth, but only
1% of the 239 non-butyrate-producing isolates consumed acetate [45]. Acetate
would then seem to be an important precursor to butyrate production, a health
benefit. However, too much acetate from bacterial production may promote
metabolic syndrome [46]. Butyrate is essential for colonic health and has been
shown to inhibit growth and induce apoptosis of colonic tumor cell lines [47],
and could therefore be used for cancer treatment. An altered gut microbiome has
been shown to increase SCFA production of acetic acid which will activate the
parasympathetic nervous system, increase glucose-stimulated insulin secretion,
increase ghrelin secretion (the hunger hormone), and contribute to hyperphagia
and obesity [48]. Therefore, acetic acid could be targeted by therapeutics to
reduce obesity.
The
data already accumulated in regards to the therapeutic use of polyols
encourages additional research into their microbiome metabolite shifts. The
shift away from production of propionate could be of extreme importance, as
research has clearly implicated propionate as a potential potentiator of A.S.D.
symptoms [49,50]. The use of polyols to treat dental diseases has proven the
safety of both xylitol and erythritol, not even considering the other positive
side-effects such as the lowering of blood pressure, triglycerides and
LDL-Cholesterol [51]. The oral microbiome of patients diagnosed with autism
spectrum disorder also demonstrate an altered oral microbiome, with an increase
in pathogens, such as, Streptococcus, that are susceptible to polyol therapy
[52]. Knowing all this should reduce hesitancy in initiating animal then
controlled human studies on microbiome shifts, metabolites shifting, and
behavioral expressions using polyols.
Conclusions
Constituents
of media, such as supplemental polyols, effect the bacterial metabolite
production of Streptococcus mutans in
vitro. Additional laboratory study is in progress testing other species,
specifically the propionic producing genus Clostridia, specifically Clostridium
bolteae and Clostridium histolyticum for the SCFA metabolite
production, and the shift in SCFA production with the addition of polyols.
Acknowledgements
We
are grateful for the support of the Goodlife Children’s Charities in providing
the guidance and advice of Dr. Derrick MacFabe. There are no conflicts of
interest to report.
References
- Clarke JK. On the
bacterial factor in the etiology of dental caries (1924) Brit J Exp Pathol 5:
141-147.
- Loesche WJ. Role
of Streptococcus mutans in human
dental decay (1986) Microbiol Rev 50: 353-380.
- Senthil Eagappan
AR, Rao VA, Sujatha S, Senthil D, Sathiyajeeva J, et al. Evaluation of salivary
nitric oxide level in children with early childhood caries (2016) Dent Res J
13: 338-341. https://doi.org/10.4103/1735-3327.187882
- Lemos JA, Palmer
SR, Zeng L, Wen ZT, Kajfasz JK, et al. The Biology of Streptococcus mutans
(2019) Microbiol Spectr 7. https://doi.org/10.1128/microbiolspec.GPP3-0051-2018
- Warinner C,
Speller C, Collins MJ and Lewis CM Jr. Ancient human microbiomes (2015) Hum
Evol 79: 125-136. https://doi.org/10.1016/j.jhevol.2014.10.016
- Bae JM.
Interpretation of the hygiene and microflora hypothesis for allergic diseases
through epigenetic epidemiology (2018) Epidemiol Health 40: e2018006. https://doi.org/10.4178/epih.e2018006
- Mäkinen KK,
Järvinen KL, Anttila CH, Luntamo LM and Vahlberg T. Topical xylitol administration
by parents for the promotion of oral health in infants: a caries prevention
experiment at a Finnish Public Health Centre (2013) Int Dent J 63: 210-224. https://doi.org/10.1111/idj.12038
- Milgrom P, Ly KA,
Tut OK, Mancl L, Roberts MC, et al. Xylitol pediatric topical oral syrup to
prevent dental caries: a double-blind randomized clinical trial of efficacy
(2009) Arch Pediatr Adolesc Med 163: 601-607.https://doi.org/10.1001/archpediatrics.2009.77
- Kuramitsu HK
andWang BY. The whole is greater than the sum of its parts: dental plaque
bacterial interactions can affect the virulence properties of cariogenic Streptococcus mutans (2011) Am J Dent
24: 153-154.
- Kressirer CA,
Smith DJ, King WF, Dobeck JM, Starr JR, et al. Scardovia wiggsiae and its
potential role as a caries pathogen (2017) J Oral Biosci 59: 135-141. https://doi.org/10.1016/j.job.2017.05.002
- Tanner AC, Kent
RL Jr, Holgerson PL, Hughes CV, Loo CY, et al. Microbiota of severe early
childhood caries before and after therapy (2011) J Dent Res 90: 1298-1305. https://doi.org/10.1177/0022034511421201
- Xiao J, Huang X,
Alkhers N, Alzamil H, Alzoubi S, et al. Candida albicans and Early Childhood
Caries: A Systematic Review and Meta-Analysis (2018) Caries Res 52: 102-112. https://doi.org/10.1159/000481833
- Matsubara VH,
Wang Y, Bandara HMHN, Mayer MPA and Samaranayake LP. Probiotic lactobacilli
inhibit early stages of Candida albicans biofilm development by reducing their
growth, cell adhesion, and filamentation (2016) Appl Microbiol Biotechnol 100:
6415. https://doi.org/10.1007/s00253-016-7527-3
- Bidossi A, De
Grandi R, Toscano M, Bottagisio M, De Vecchi E, et al. Probiotics Streptococcus salivarius 24SMB and Streptococcus oralis 89a interfere with
biofilm formation of pathogens of the upper respiratory tract (2018) BMC
infectious diseases 18: 653. https://doi.org/10.1186/s12879-018-3576-9
- Hillman JD,
Socransky SS and Shivers M. The relationships between streptococcal species and
periodontopathic bacteria in human dental plaque (1985) Arch Oral Biol 30:
791-795.
- Hedayati-Hajikand
T, Lundberg U, Eldh C and Twetman S. Effect of probiotic chewing tablets on
early childhood caries-a randomized controlled trial (2015) BMC Oral Health 15:
112. https://doi.org/10.1186/s12903-015-0096-5
- Hillman JD and
Socransky SS. The theory and application of bacterial interference to oral
diseases (1989) Myers HM (ed) Karger, Basel, Switzerland, 1-17.
- Zahradnik RT,
Magnusson I, Walker C, Mcdonnell E, Hillman CH, et al. Preliminary assessment
of safety and effectiveness in humans of ProBiora3, a probiotic mouthwash
(2009) J Applied Micro 107: 682-690.
- Hillman JD,
McDonnell E, Hillman CH, Zahradnik RT, Soni MG. Safety assessment of ProBiora3,
a probiotic mouthwash; subchronic toxicity study in rats (2009) Int J
Toxicology 28: 357-367. https://doi.org/10.1177/1091581809340705
- Cannon M. A
Review of probiotic therapy in preventive dental practice (2011) Probiotics
Anti-microb Proteins 3: 63-67. https://doi.org/10.1007/s12602-011-9072-9
- Cannon M, Trent
B, Vorachek A, Kramer S and Esterly R. Effectiveness of CRT at measuring the
salivary level of bacteria in caries prone children with probiotic therapy
(2013) J Clin Pediatr Dent 38: 55-60. https://doi.org/10.17796/jcpd.38.1.b481624264142082
- Cannon M.
Clinical Application of Probiotic Therapy (2011) Inside Dentistry 7: 112-113.
- Janakiram C,
Deepan Kumar CV and Joseph J. Xylitol in preventing dental caries: A systematic
review and meta-analyses (2017) J Nat Sci Biol Med 8: 16-21. https://doi.org/10.4103/0976-9668.198344
- Park E, Sam Na H,
Min Kim S, Wallet S, Cha S, et al. Xylitol, an anticaries agent, exhibits
potent inhibition of inflammatory responses in human THP-1-derived macrophages
infected with porphyromonas gingivalis (2014) J Periodontol 85: e212–e223. https://doi.org/10.1902/jop.2014.130455
- Nayak PA, Nayak
UA and Khandelwal V. The effect of xylitol on dental caries and oral flora
(2014) Clin Cosmet Investig Dent 6: 89-94. https://doi.org/10.2147/CCIDE.S55761
- Söderling E,
Isokangas P, Pienihäkkinen K and Tenovuo J. Influence of maternal xylitol
consumption on acquisition of mutans streptococci by infants (2000) J Dent Res
79: 882-887. https://doi.org/10.1177/00220345000790031601
- Falony G, Honkala
S, Runnel R, Olak J, Nõmmela R, et al. Long-term effect of Erythritol on dental
caries development during childhood: a posttreatment survival analysis (2016)
Caries Res 50: 579-588. https://doi.org/10.1159/000450762
- Mansour TR, Alam
Y, Dahbour L, Alnemari A, Jumaa M, et al. Streptococcus
Mutans: A potential risk factor in recurrent hemorrhagic stroke (2017)
Cureus 9: e1264. https://doi.org/10.7759/cureus.1264
- Runnel R, Mäkinen
KK, Honkala S, Olak J, Mäkinen PL, et al. Effect of three-year consumption of
erythritol, xylitol and sorbitol candies on various plaque and salivary
caries-related variables (2013) J Dent 41: 1236-1244. https://doi.org/10.1016/j.jdent.2013.09.007
- Hojo S, Komatsu
M, Okuda R, Takahashi N and Yamada T. Acid profiles and pH of carious dentin in
active and arrested lesions (1994) J Dent Res 73: 1853-1857. https://doi.org/10.1177/00220345940730121001
- Falony G, Honkala
S, Runnel R, Olak J, Nõmmela R, et al. Long-term effect of Erythritol on dental
caries development during childhood: a posttreatment survival analysis (2016)
Caries Res 50: 579-588. https://doi.org/10.1159/000450762
- MacFabe DF, Cain
DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, et al. Neurobiological effects
of intraventricular propionic acid in rats: possible role of short chain fatty
acids on the pathogenesis and characteristics of autism spectrum disorders
(2007) Behav Brain Res176:149-169. https://doi.org/10.1016/j.bbr.2006.07.025
- Shultz SR, MacFabe DF, Ossenkopp KP, Scratch S, Whelan J, et al. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism (2008) Neuropharmacology 54: 901-911. https://doi.org/10.1016/j.neuropharm.2008.01.013
- Shultz SR,
Macfabe DF, Martin S, Jackson J, Taylor R, et al. Intracerebroventricular
injections of the enteric bacterial metabolic product propionic acid impair
cognition and sensorimotor ability in the Long-Evans rat: further development
of a rodent model of autism (2009) Behav Brain Res 200: 33-41. https://doi.org/10.1016/j.bbr.2008.12.023
- Foley KA,
Kavaliers M, MacFabe DF, Ossenkopp KP. Systemic treatment with the enteric
bacterial metabolic product propionic acid results in reduction of social
behavior in juvenile rats: Contribution to a rodent model of autism spectrum
disorder (2019) Dev Psychobiol. https://doi.org/10.1002/dev.21825
- MacFabe DF, Cain
NE, Boon F, Ossenkopp KP and Cain DP. Effects of the enteric bacterial
metabolic product propionic acid on object-directed behavior, social behavior,
cognition, and neuro-inflammation in adolescent rats: Relevance to autism
spectrum disorder (2011) Behav Brain Res 217: 47-54. https://doi.org/10.1016/j.bbr.2010.10.005
- Frye RE, Nankova
B, Bhattacharyya S, Rose S, Bennuri SC, et al. Modulation of immunological
pathways in autistic and neurotypical lymphoblastoid cell lines by the enteric
microbiome metabolite propionic acid (2017) Front Immunol 8: 1670. https://doi.org/10.3389/fimmu.2017.01670
- Rose S, Bennuri
SC, Davis JE, Wynne R, Slattery JC, et al. Butyrate enhances mitochondrial
function during oxidative stress in cell lines from boys with autism (2018)
Translational Psychiatry 8: 42.
- Lin HV, Frassetto
A, Kowalik EJ Jr, Nawrocki AR, Lu MM, et al. Butyrate and propionate protect
against diet-induced obesity and regulate gut hormones via free fatty acid
receptor 3-independent mechanisms (2012) PloS one 7: e35240. https://doi.org/10.1371/journal.pone.0035240
- Dengate S and
Ruben A. Controlled trial of cumulative behavioural effects of a common bread
preservative (2002) J Paediatr Child Health 38: 373-376.
- McNabney SM and
Henagan TM. Short chain fatty acids in the colon and peripheral tissues: a
focus on butyrate, colon cancer, obesity and insulin resistance (2017)
Nutrients 9: 1348. https://doi.org/10.3390/nu9121348
- Cummings JH and
MacFarlane GT. The colonic flora, fermentation and large bowel digestive
function. in The large intestine: physiology, pathophysiology, and disease
(1991) Philips SF, Pemberton JH and Shorter RG (ed) Raven Press, New York, USA,
51-91.
- Szylit O and
Andrieux C. Physiological and pathophysiological effects of carbohydrate
fermentation (1993) World Rev Nutr Diet 74: 88-122.
- Hague A, Singh B
and Paraskeva C. Butyrate acts as a survival factor for colonic epithelial
cells: further fuel for the in vivo versus in vitro debate (1997)
Gastroenterology 112: 1036-1040.
- Barcenilla A,
Pryde SE, Martin JC, Duncan SH, Stewart CS, et al. Phylogenetic Relationships
of Butyrate-Producing Bacteria from the Human Gut (2000) Appl Environ Microbiol
66:1654-1661.
- Chambers ES,
Preston T, Frost G and Morrison DJ. Role of Gut Microbiota-Generated
Short-Chain Fatty Acids in Metabolic and Cardiovascular Health (2018) Current
Nutrition Reports 7: 198-206. https://doi.org/10.1007/s13668-018-0248-8
- Bultman SJ.
Molecular pathways: gene-environment interactions regulating dietary fiber
induction of proliferation and apoptosis via butyrate for cancer prevention
(2014) Clinical Cancer Research: an official journal of the American
Association for Cancer Research, 20: 799-803. https://doi.org/10.1158/1078-0432.CCR-13-2483
- Perry RJ, Peng L,
Barry NA, Cline GW, Zhang D, et al, Acetate mediates a microbiome–brain–β-cell
axis to promote metabolic syndrome (2016) Nature 534: 213-217. https://doi.org/10.1038/nature18309
- Frye RE, Rose S,
Slattery J and MacFabe DF. Gastrointestinal dysfunction in autism spectrum
disorder: the role of the mitochondria and the enteric microbiome (2015) Microb
Ecol Health Dis 26. https://doi.org/10.3402/mehd.v26.27458
- Li Q, Han Y, Dy
ABC and Hagerman RJ. The Gut Microbiota and Autism Spectrum Disorders (2017)
Front Cell Neurosci 11:120. https://doi.org/10.3389/fncel.2017.00120
- Ur-Rehman S, Mushtaq Z, Zahoor T, Jamil A and Murtaza MA. Xylitol: a review on bioproduction, application, health benefits, and related safety issues (2015) Crit Rev Food Sci Nutr 55: 1514-1528. https://doi.org/10.1080/10408398.2012.702288
- Qiao Y, Wu Y, Feng Y, Zhou Z, Chen L, et al. Alterations of oral microbiota distinguish children with autism spectrum disorders from healthy controls (2018) Sci Rep 8: 1597. https://doi.org/10.1038/s41598-018-19982-y
*Corresponding author:
Mark
Cannon, Feinberg School of Medicine, Northwestern University, USA, E-mail: drmarkcannon@outlook.com
Goudarzi S Habibi, Kabat B, Cannon M, Gashkoff M and Zurek R. Pilot study of the
SCFA Headspace Analysis of Streptococcus
mutans Metabolites in Media with and without Polyols (2020) Edel J Biomed
Res Rev 2: 24-30.
Keywords
Streptococcus mutans, Polyols, Gut microbiome.


 PDF
PDF