Introduction
Beer
is obtained by fermentation of glucose molecules of plant origin. In other
words, beer is an alcoholic beverage obtained by transformation of starchy
substances by enzymatic and microbiological means. The aim of this review is to
show different organic reactions, which take place during the production of
beer with emphasis on the reactivity of organic compounds as well as the
reaction mechanisms. It is important to mention that the understanding of a
reaction mechanism helps to select starting materials and predict the formation
of a desired product or the knowledge of a reaction mechanism permits to set up
appropriate conditions in order to generate a targeted product.
Chemical Ingredients
Water
Water
in the beer must be free of organic and inorganic pollutants or any undesirable
products such as bacteria, sediments, halogenated aromatic organic compounds. Water
contains mineral salts, which must be controlled. Water is necessary for
brewing as well as for cleaning and rinsing equipment used in the brewery.
Spring or well water is desirable because it contains a small amount of mineral
salts, but it has to be checked regularly in order to verify its purity. It is
very important to mention that the quality of beer depends on the chemical
characteristics of water [1,2,3].
Malt
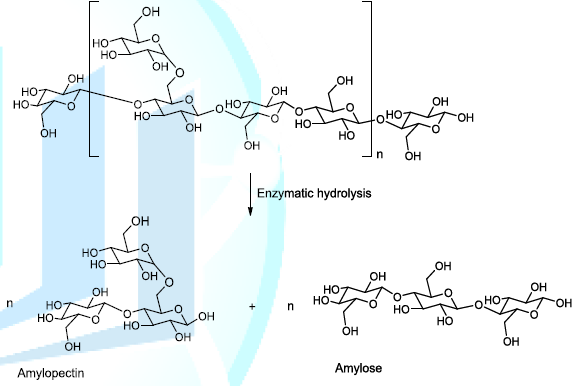
Malt is made from cereals particularly barley, which is most often utilized in brewery. In this regard, at the malt house, cereals are placed in conditions which allow their germination and then, they are dried. This kind of technology is called malting, a technology that sets free enzymes, which hydrolyse the starch to produce amylopectin (branched polymer of glucose molecules) and amylose (linear chain composed of glucose molecules) into the reaction medium (Scheme 1). The plausible enzymatic mechanism of starch hydrolysis has been recently reported in the literature [4,5].
Yeasts
Yeasts are microscopic unicellular fungi that live and proliferate by consuming sugars. In anaerobic conditions, yeasts convert sugars into ethanol and carbon dioxide. This kind of conversion is called fermentation. This chemical process is the basis for the production of any alcoholic beverage. During fermentation, the yeasts also produce a variety of aromatic substances, which give the beer its own characteristic [5,6].
Caramelization
Caramelization is a non-enzymatic oxidation reaction extensively utilised in cooking to obtain the natty flavor and brown colour. During caramelization, volatile organic substances are released, and that allows a characteristic flavor of the caramel. Indeed the reaction involves the loss of water in the form of vapor, and the cleavage of glucose (Scheme 2, reaction 1). The thermal degradation of sugar, during the caramelization process, involves diverse reactions such as the intra molecular rearrangement or Lobry-de Bruyn-van Ekenstein Rearrangement. This step is followed by beta elimination of a molecule of water (dehydration reaction) and dicarbonyl group cleavage as well (Scheme 2, reaction 2,3) [7-10].
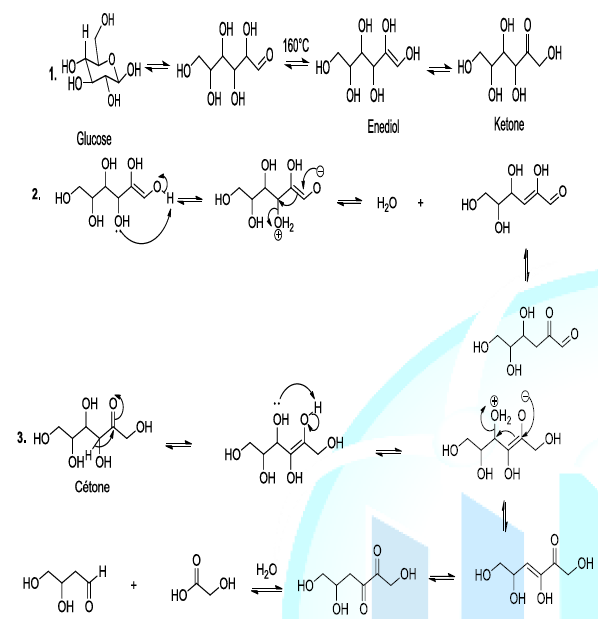
Scheme 2: Thermal decomposition of glucose
Most importantly, the enolization of glucose is a pivot reaction because it initiates the degradation process. Indeed, the organic compounds generated during the thermal decomposition of glucose can subsequently react to produce carboxylic compounds, and oxygenated heterocyclic substances via aldol condensation. From all the sugar degradation reactions, the strategic intermediate compounds from thermal caramelization are dicarbonyl compounds. These kind of compounds lead not only to the formation of caramel coloration, but also they generate volatile products, which are typical of the caramel flavor [7-10].
Humulus
lupulus
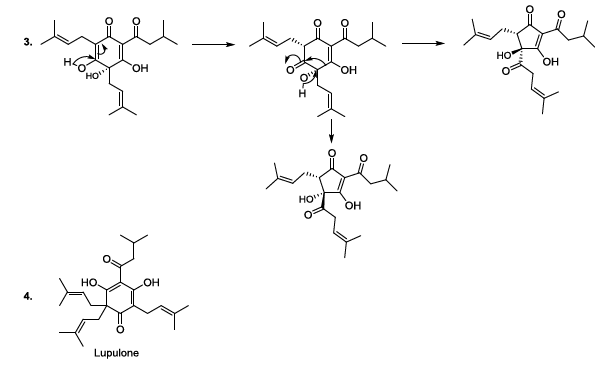
Hop or Humulus lupulus L. is a climbing plant is the Cannabaceae family. The female plants produce flowers in the form of small cones made up of leaflets. Underneath these small leaves are tiny yellow glands with lupulin, which contains better resins and aromatic essential oils. Therefore, the hop gives the beer a bitter taste and a characteristic aroma according to the used technology. It contributes to the retention of foam, and to the shelf life of the beer. It can be utilized as a cone, its coolest form or as a compressed granule, which is practical and durable. In addition, the tannic substances contained in the leaflets allow the hop to play the role of preservative and natural clarifier of the beer [11-13]. During the beer production, lupulin is transformed to produce bioactive humulone and lupulone (Scheme 3). The antioxidant and antibacterial Lupulone as well as humulone both participate to the preservation of beer. Under thermal conditions, Humulone is, at its turn, converted to isohumulone, an antibacterial with a bitter flavor (Scheme 3) [14-22].
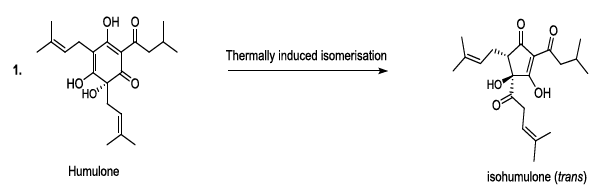
Scheme 3a: Thermally induced isomerisation
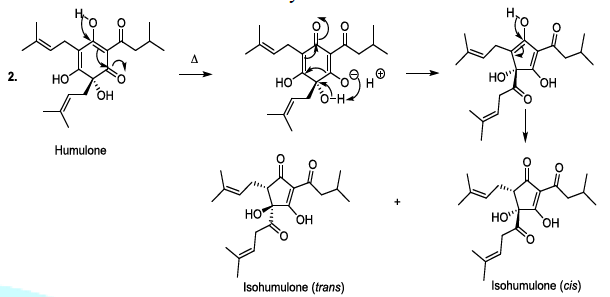
Scheme 3b: Thermally induced isomerisation

Scheme 4: Isohumulone degradation mechanism
The isomerization of humulone can also be carried out in mild alkaline conditions, and in this case, the plausible mechanism involves the formation of a single anion (Scheme 5). This sequence is followed by the formation of a ketone group in stereospecific manner, and the cyclic contraction to furnish trans and cis isohumulone in ratio of 32/68 [14-22]. It has been reported that in harsh alkaline conditions, two anions are formed, and this results in the production of two trans and cis isohumulone in ratio of 50/50 [14-22].
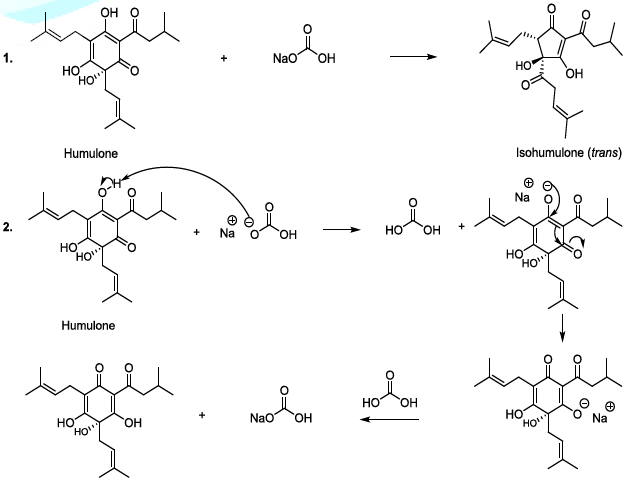
Scheme 5: Isomerization of humulone mechanism
Oxygen
Oxygen alters rapidly the flavorof the beer. This means that oxygen is the initiator of chemical reactions that produce oxygenated reactive entities. Indeed, Oxygen in the ground state is normally stable and it cannot easily react with organic compounds. Nevertheless, in the presence of Fe(II) or Cu(I) in beer, the oxygen can capture an electron to form a radical anion, which can remove a proton to generate a more reactive hydroxyl radical. This later can also react with iron (II) or copper (I) to produce a peroxide anion. In beer, the peroxide anion is transformed into hydrogen peroxide. The hydroxyl radical can be produced from the hydrogen peroxide (Fenton reaction) or from oxygen (radical anion) by metallic induction (Scheme 6, reaction 1-4) (Haber-Weiss reaction) [23-26].
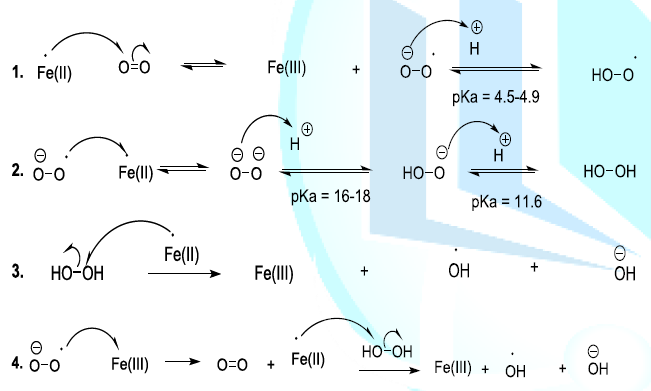
Scheme 6: Generation of oxygenated reactive entities
Oxygenated entities degrade organic constituents of beer, and correspondingly, they promote the aging or deterioration of the beer quality (Scheme 7) [23-26].
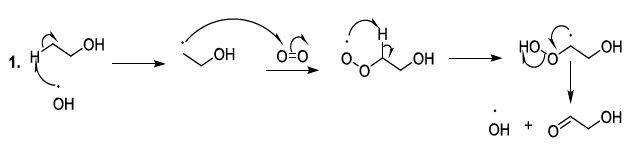
Scheme 7a: Degradation of organic constituents of beer
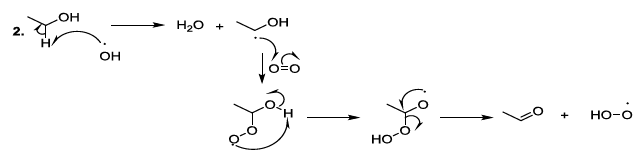
Scheme 7b: Degradation of organic constituents of beer mechanism
Fatty
acids
Reactive oxygenated species also react with lipids or fatty acids. Indeed, the oxidation of lipids starts with the removal of a hydrogen atom by free radicals specially hydroxyl radicals or peroxides. In the case of linoleic acid, the hydrogen situated upon carbon 11 is a hydrogen atom that is easy to be removed because it is activated by the two neighboring double bonds. It has been reported that hydroperoxide acids can be decomposed, in acidic conditions, to produce diverse volatile compounds, and in this perspective, several reaction mechanisms have been proposed whose the ionic mechanism (Scheme 8, reactions 1,2) [23-26].
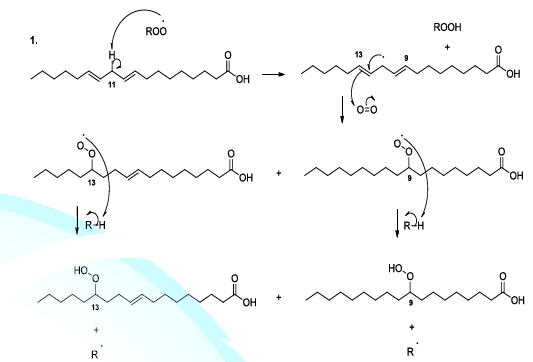
Scheme 8a: Fatty acid oxidation mechanism
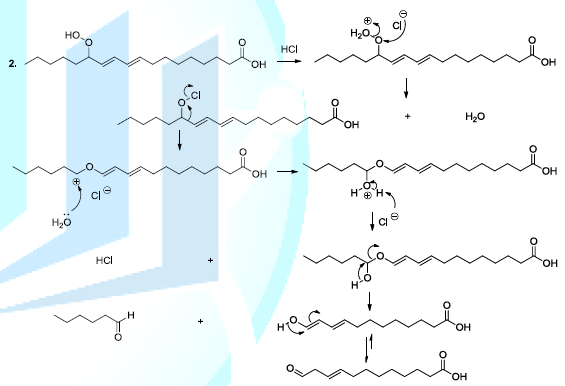
Scheme 8b: Fatty acid oxidation mechanism
Carbonyl compounds
Experimental observations stated that aldol condensation of carbonyl compounds is plausible under mild conditions in beer during storage, and in this regard, amino acids behave as bases, nucleophiles, organic catalysts, and they contribute to the formation of imine intermediate products. This kind of process can lead to the production of carbonyl compounds after the hydrolysis of the corresponding imines (Scheme 9) [23-26].
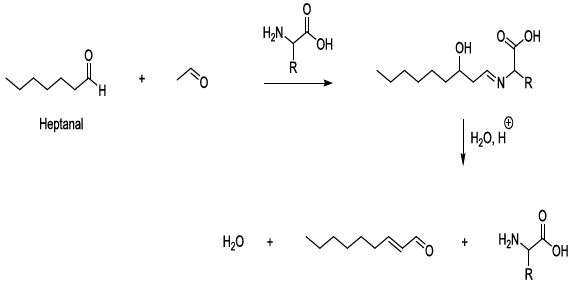
Scheme 9: Catalysis of aldol condensation
Conclusion
This
review has shown enzymatic and non-enzymatic organic reactions occurring in
beer production in order to better understand, for example, why oxygen has to
be prevented to get in contact with beer. This is very important so that the
quality of beer remains optimal. The knowledge of organic reactions also helps
to understand the importance of preventing oxygen during fermentation because
it will destroy the enzymatic transformation of sugars due to the presence of
yeasts in the production of alcoholic beverage. Organic reactions play a
notable role because the aging of beer can be preserved in using additives such
as ascorbic acid. In fact, beyond the technology of measurements on devices to
reduce the influence of atmospheric oxygen during bottling, ascorbic acid has
proven to be a suitable oxygen reducer or a reliable antioxidant during beer
brewing.
References
- Mwene-Mbeja TM. Deforestation
and Chemical Water Quality. Eur. J. Sci. Res (2017) 144: 129-142.
- Dekov
VM, Komy Z, Araujo F, Van Put A and Van Grieken. Chemical composition of
sediments, suspended matter, river water and ground water of the Nile (Aswan-Sohag
traverse) (1997) Sci Total Environ 201: 195-210. https://doi.org/10.1016/s0048-9697(97)84057-0
- Hai ZC.
International Conference on New Technology of Agricultural (2011) 643.
- Evans
DE, Redd k, Haraysmow SE, Elvig N, Metz N, et al. The influence of malt quality
on malt brewing and barley quality on barley brewing with ondea pro, compared
by small-scale analysis (2014) J Am Soc Brew Chem 72: 192-207.
- Mwene-Mbeja
TM, Dufour A, Lecka J, Kaur SB and Vanneechhaute C. Enzymatic reactions in the
production of biomethane from organic waste (2020) 132: 109410. https://doi.org/10.1016/j.enzmictec.2019.109410
- Bokulich
NA and Bamforth CW. The microbiology of malting and brewing (2013) Microbiol Mol
Biol Rev 77: 157-172.
- Murzin
DY, Murzina EV, Aho A, Kazakova MA, Selyutin A, et al. Aldose to ketose
interconversion: galactose and arabinose isomerization over heterogeneous
catalysts (2017) Catal Sci Technol 7: 5321. https://doi.org/10.1039/c7cy00281e
- Carraher
JM, Fleitman CN and Tessonnie JP. kinetic and mechanistic study of glucose
isomerization using homogeneous organic brønsted base catalysts in water (2015)
ACS Catal 5: 3162-3173. https://doi.org/10.1016/0308-8146(94)90188-0
- Kroh
LW. Caramelisation in food and beverages (1994) Food Chem 51: 373-379.
- Lee
GC and Lee CY. Inhibitory effect of caramelisation products on enzymic browning
(1997) Food Chem 60: 231-235. https://doi.org/10.1016/S0308-8146(96)00325-1
- Karacin
M, Hudcova T, Jelinek L and Dostalek P. Biologically Active Compounds from Hops
and Prospects for Their Use (2016) Compr Rev Food Sci Food Saf 15: 542.
- Hrnčič
MK, Španinger E, Košir IJ, Knez Z and Bren U. Hop Compounds: Extraction
Techniques, Chemical Analyses, Antioxidative, Antimicrobial, and
Anticarcinogenic Effects (2019) Nutrients 11: 257. https://doi.org/10.3390/nu11020257
- Mishra
AK, Kocábek T, Nath VS, Awasthi P, Shrestha A, et al. Dissection of
Dynamic Transcriptome Landscape of Leaf, Bract, and Lupulin Gland in Hop
(Humulus lupulus L.) (2020) Int J Mol Sci 21: 233. https://doi.org/10.3390/ijms21010233
- Dybowski
MP, Typek R, Bernacik K and Dawidowicz AL. Isomerization of bitter acids during
the brewing process (2015) Annales UMCS 70: 137-144.
- Sharpe
FR and Ormrod IHL. Fast isomerisation of humulone by photo‐reaction:
preparation of an hplc standard (1991) J Inst Brew 97: 33-37.
- Verzele
M, Boven MV. The Isomerization Mechanism of Humulone (1971) Bull Soc Chim
Belges 80: 677. https://doi.org/10.1002/bscb.19710800538
- Urban
J, Dahlberg CJ, Carroll BJ and Kaminsky W. Absolute Configuration of Beer's
Bitter Compounds (2013) Angew Chem Int Ed Engl 52: 1553-1555.
- Fedorova
OS, Ryvkina LS and Berdnikov VM. Mechanism of ascorbic acid oxidation by
molecular oxygen in aqueous pyridine catalyzed by CO2+, Ni2+, Mn2+ and Zn2+
(1980) Reaction Kinet Catal Lett 15: 67-72.
- Vanderhaegen
B, Neven H, Verachtert H and Derdelinckx G. Sensory Characterization of
Commercial Lager Beers and Their Correlations with Iso-α-Acid Concentrations
(2006) Food Chem 95: 357-381.
- Intelmann
D and Hofmann T. On the autoxidation of bitter-tasting iso-alpha-acids in beer
(2010) Agric Food Chem 58: 5059-5067.
- Deana AA, Stokker GE, Schultz EM, Smith RL, Cragoe Jr, et al. 2-(Aminomethyl)phenols, a new class of saluretic agents. 5. Fused-ring analogs (1983) J Med Chem 26: 580-585.
- Huvaere
K, Sinnaeve B, Bocxlaer VJ and Keukeleire DD. Photooxidative degradation of
beer bittering principles: product analysis with respect to light struck flavor
formation (2004) Photochem Photobiol Sci 3: 854-858. https://doi.org/10.1039/b403666b
Corresponding author
Topwe Milongwe Mwene-Mbeja, Department of Chemistry, Faculty of Science, University of Lubumbashi, Lubumbashi, Democratic Republic of the Congo, Hydro-Quebec Institute for Environment, Development and Society at Laval University, Quebec, Canada, Email: topwe.mwenembeja@unilu.ac.cd; topwe@hotmail.caCitation
Mwene-Mbeja TM. Chemistry of organic compounds in the beer production (2020) Edelweiss Food Sci Tech 1: 32-35.Keywords
Chemistry, Organic Compound, Mechanism and Beer


 PDF
PDF