Introduction
Huntington’s
Disease (HD) is a neurodegenerative condition characterized cellularly by the
loss of neurons in the basal ganglia and clinically by uncontrolled movements,
emotional problems, and loss of cognition [1]. HD is an autosomal dominantly
inherited disorder and although prevalence rates range widely depending on
geography and ethnicity, it is thought to affect more than 50,000 people in the
United States and Europe alone [2]. The underlying genetic cause is a
trinucleotide CAG expansion in the huntingtin gene (HTT), discovered as a
genetic marker by James Gusella from Massachusetts General Hospital in 1983,
which results in the production of a mutant Huntingtin Protein (mHTT) with a
toxic poly-glutamine (polyQ) tract [3,4]. However, despite decades of research
and clinical trials, no successful therapy has yet been developed. The
“typical” onset of HD is between the ages of 40-50, but up to 15% of cases have
very late onset and don’t show clinical symptoms until after the age of 60 [5].
In
a recent meta-analysis of studies investigating cases of Late-onset HD (LoHD),
more than 90% of patients had CAG repeat lengths of ≤ 44 [6]. One of the more
interesting observations in HD is that while there is a well-known correlation
between the length of the polyQ repeat tract and the onset and severity of the
disease, there is substantial variability within individual patients. For
example, in patients with mid-range repeat lengths (defined here as between 40
and 50), disease onset can vary by 60 years in any individual patient [5]. This
means that many patients who are carriers of polyQ tracts that predispose to
the development of the disease can live for decades in a “presymptomatic” state
[7]. What controls the onset of clinical symptoms remains currently unknown and
complicates the prognostic evaluation of HD patients. Although historically
considered a monogenic disease; genetic, environmental and unknown factors can
influence disease onset and progression. Many different technologies have been
used to look at the molecular changes underlying disease progression in HD
including gene expression, proteomics, metabolomics, network analysis, genomics
and Single Nucleotide Polymorphism (SNP) profiling) [8-13]. Recently,
epigenetic approaches have emerged as a promising new tool for assessing
pathology-related changes [14]. Most epigenetic studies in HD have focused on
looking at genome wide histone modifications (acetylation, methylation) or
histone modifications at specific loci related to HD [15-17]. While these
approaches have provided interesting insight into the disease, they have
yielded often conflicting results and shown inconsistencies between mouse
models and human disease [14,18,19]. As such, a consensus picture of epigenetic
deregulation in HD using histone modification readouts has yet to materialize.
However, not all molecular mechanisms associated with epigenetic regulation
have been assessed in the context of HD. An important aspect of epigenetic
regulation is at the level of 3-dimensional (3D) genomic architecture [20].
The
3D organization of the genome reflects the heterogeneous effects of external
environmental cues and inputs and can be empirically measured by the assessment
of chromosome conformations or when several conformations are measured
concomitantly, a Chromosome Conformation Signature (CCS) [21]. CCSs can be
thought of as the molecular barcode that gives a readout of the epigenetic
landscape of a given cellular population [22,23] To date, the evaluation of
CCSs in HD has remained unexplored. Given the central role of mHTT in the
development of HD, we hypothesized that regulatory difference in genomic
architecture at the HTT locus may exist between diseased individuals and
healthy, unaffected controls.
We
used EpiSwitch®, an established proprietary industrial platform for
monitoring CCSs, to assess chromatin architecture differences between
presymptomatic and symptomatic HD patients and healthy, unaffected individuals.
EpiSwitch® readouts provide high resolution, reliable and high
throughput detection of CCSs while simultaneously meeting the high bar of
industry standards for quality control [21]. As such, this technology
represents a powerful tool for screening, evaluation and monitoring of CCS in
human disease [24]. This platform has been successfully utilized as a biomarker
modality to stratify patients in the context of a variety of other diseases [25-33],
including as a non-invasive blood based biomarker for neurodegenerative
conditions [34,35].
Methods
Sample
Collection
All
samples were obtained from National BioService, LLC. In total, 20 samples were
used in this study; 10 Healthy Control (HC) samples (CAG repeats, n<35), and
10 HD samples (CAG repeats, n>39). For the HD samples, 7 were from
symptomatic patients (HD-Sym) and 3 were from presymptomatic patients who had a
diagnosis of HD but did not yet show any clinical symptoms (HD-Pre). One HD
patient was taking tetrabenazine and one patient was taking sertraline. All
samples were negative for human immunodeficiency virus, hepatitis B virus, hepatitis
C virus and syphilis (Supplemental Table
1).
Study
Design
We wanted to identify chromosome conformations that differed between healthy controls (low CAG), presymptomatic HD patients (high CAG, no disease manifestation) and symptomatic HD patients (high CAG, disease manifestation). We focused on a ~225 kb region surrounding the HTT locus from (chr4: 3,033,588 to 3,258,170 as annotated in hg38) for our analysis. Using the CAG repeat expansion tract in exon 1 of HTT (chr4: 3,054,162 to 3,095,930) as the anchor point (“Anchor”), we defined five genomic zones surrounding the Anchor to look at chromosome conformations that varied between sample groups (Figure 1 and Supplemental Table 2). These Zones were chosen based on: the presence of potential EpiSwitch anchoring sites; the presence of known disease related-SNPs (HD and other diseases); and the enrichment of known histone modification sites (H3K4me3, H3K36me3, and H3K27ac) in HD as found in the GWASdvV2 database (http://jjwanglab.org/gwasdb) (Figure 1).
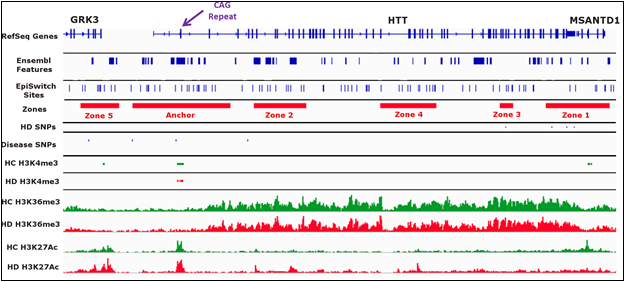
Figure 1: Definition ofthe Anchor point and Zones for this study.
Chromosome
Conformation Identity
A
search of the NCBI: GEO database for previously reported HD epigenetic data was
performed in February 2018 [36]. Peak called ChIP-seq data for H3K4me3 from 12
(6 HD and 6 control samples) post-mortem prefontal cortex brain samples (bed
format) was obtained (GSE68952) [37]. In addition, Bigwig tracks of ChIP-seq
data for H3K27ac and H3K36me3 from HD iPSC-derived neural cell lines and
control cell lines were also downloaded (GSE95342) [38]. The data tracks were
loaded into the Integrative Genome Viewer (IGV) (41) alongside the EpiSwitch®
and reference sequence annotations [39]. Both visual and programmatic
(BEDtools) comparisons were performed on the HTT locus to identify the 5 Zones
of interest.
Oxford
BioDynamics proprietary EpiSwitch® software was used to identify
high probability chromatin folding interactions with one “end” occurring in the
Anchor zone proximal to the CAG repeats and the other in any of the 5 Zones of
interest. A total of 61 interactions matched these criteria, and for practical
reasons, 20 interactions were selected to cover interactions between the Anchor
site and all the Zones of interest. Oxford BioDynamics automated primer design
application was used to design oligonucleotide pairs that amplified the
expected DNA sequence caused by the interaction when subjected to the
chromosome conformation capture assay.
3C
and PCR
Chromosome
conformation capture and detection by PCR were performed as described
previously [29,40-42]. Chromatin with intact chromosome conformations from 50
µl of blood sample from each patient sample was extracted using the EpiSwitch®
assay following the manufacturer's instructions (Oxford BioDynamics Plc).
Quality control on all samples was done using the detection of a chromatin loop
at the MMP1 locus, a historical internal control for 3C analysis [29]. Pooled
3C libraries for each of the sample types were generated to provide a
generalized population sample for each of the sample subgroups. Real-time PCR
was performed with SYBR green with the CFX-96 (Bio-Rad) machine to identify the
interactions with differing PCR product detection patterns between the sample
types.
Oligonucleotides
were tested on control templates to confirm that each primer set was working
correctly. In line with Royal Forensic Protocol for PCR detection the final
nested PCR was performed on each sample in triplicates for the follow up data
on individual HD patients. This procedure permitted the detection of limited
copy-number templates with higher accuracy. All PCR amplified products were
monitored on the LabChip® GX from Perkin Elmer, using the LabChip
DNA 1K Version2 kit (Perkin Elmer) and internal DNA markers were loaded on the
DNA chip according to the manufacturer’s protocol using fluorescent dyes.
Fluorescence
was detected by laser and electropherogram read-outs translated into a
simulated band on gel picture using the instrument software. The threshold of
detection for the instrument was set by the manufacturer from 30 fluorescence
units and above.
Statistical
Analysis
Data
analysis was performed in R (language and environment for statistical
computing). This included stats and dplyr packages for t-tests and R-squared
analysis and a ggplot2 package for boxplots and regression plots.
Results
Patient
Clinical Characteristics
HC and HD samples were age (average 36.9 for HC and 35.3 for HD) and sex matched (½ male and ½ female), with the majority (70%) of HD cases being symptomatic (Table 1).
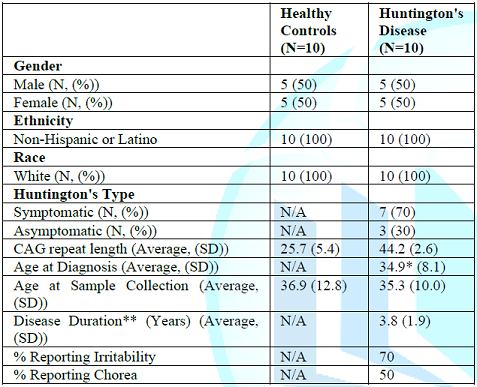
Table 1: Clinical characteristics of the samples used in this study.
All samples were from Non-Hispanic or Latino Whites. Average CAG repeats lengths were 25.7 for HC and 44.2 for HD (Table 1, Figure 2). There was no statistical difference in CAG repeat length between HD-Pre and HD-Sym. The average age at diagnosis for HD samples was 35.3 and the average disease duration was 3.8 years with 7 out of 10 patients reporting symptoms of irritability, chorea, or both.
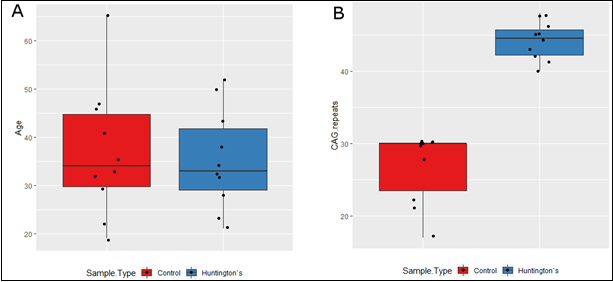
Figure 2: Clinical characteristics of the samples used in this study.
Chromosome
Conformations in HC, HD-Pre and HD-Sym
Of
the 20 interactions that were evaluated (Supplemental
Table 3), we identified nine informative interactions. We identified two
constitutive interactions and seven conditional interactions which were present
in HD, but not healthy controls. Three of the seven conditional interactions
were present only in HD-Sym, and absent in HD-Pre.
Constitutive
Conformations
All samples passed internal QC analysis for the MMP1 interaction (Supplemental Figures 1 and 2). Two constitutive (identified in all samples) chromatin loops were identified. Both loops were between the Anchor and Zone 2 with the first loop spanning 28 kb and the second loop spanning 34 kb (Figure 3).
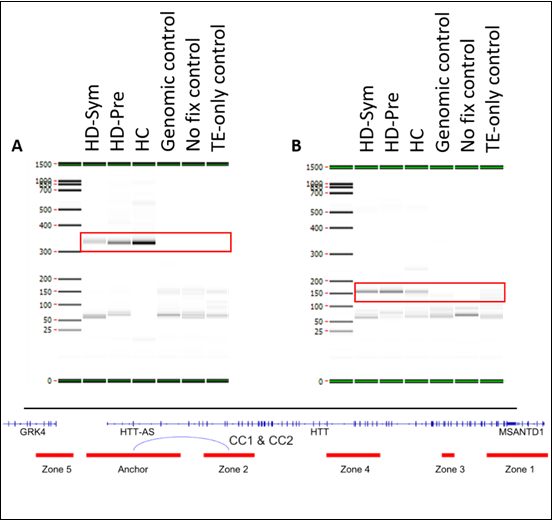
Figure 3: Summary of constitutive interactions.
Conditional
Conformations
We
identified seven conditional chromosome conformations that could discriminate
between the different patient subgroups evaluated in this study. Specifically,
we identified two chromosome interactions that were present in HC, but absent
in all HD samples (Figure 4).
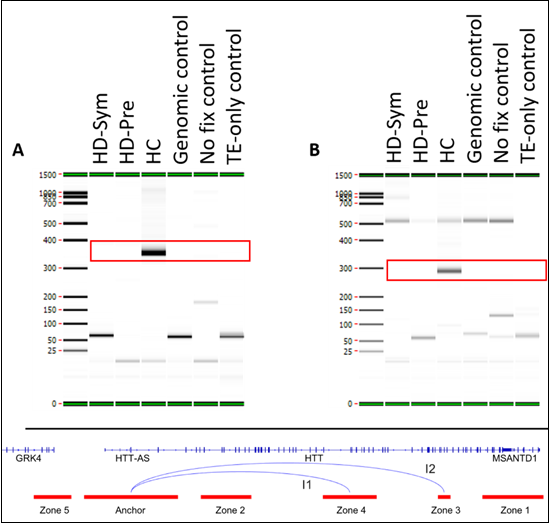
Figure 4: Summary of conditional interactions-Healthy Controls.
The first interaction (I1) spanned the Anchor and Zone 4 and covered 77 kb while the second interaction (I2) spanned the Anchor and Zone 3 and covered 140 kb. We also identified two chromosome interactions that were present in HC and HD-Pre, but absent in HD-Sym samples. Both interactions (I3 and I4) spanned the Anchor and Zone 4 and covered 92 kb and 104 kb, respectively (Figure 5).
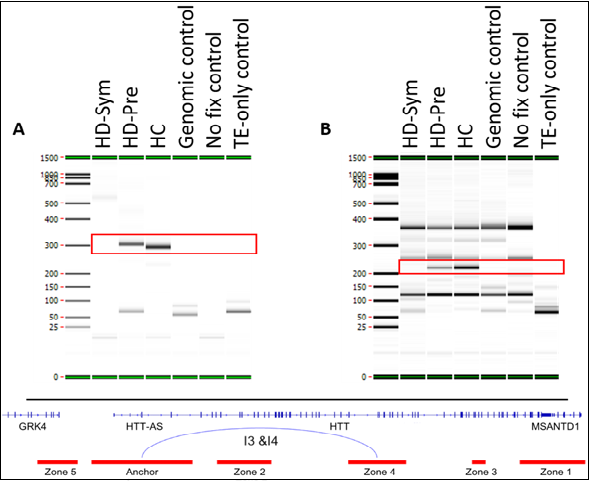
Figure 5: Summary of conditional interactions- Huntington’s Disease Negative.
Last,
we identified three chromosome interactions that were present in HD-Sym
samples, but absent in HD-Pre and HC samples. The first interaction (I5)
spanned the Anchor and Zone 3, covering 122kb. Interestingly, this interaction
included a SNP (rs362331) known to be a factor in the predisposition to develop
HD. The second and third interactions (I6 and I7) spanned the Anchor and Zone 1
and covered 185 kb and 174 kb, respectively (Figure 6). Last, we tested the absence or presence of all
conditional interactions in individual HD samples. In the HD-Sym samples, we
found the presence of at least one of the conditional markers (I5, I6 and I7)
in six out of seven samples (Figure 7).
The odds ratios of each interaction being associated with symptomatic HD
presentation were 72, 9 and 16 for I5, I6 and I7, respectively. A summary of
all the interactions that were evaluated in this study are shown in Figure 8 and Supplemental Figure 3.
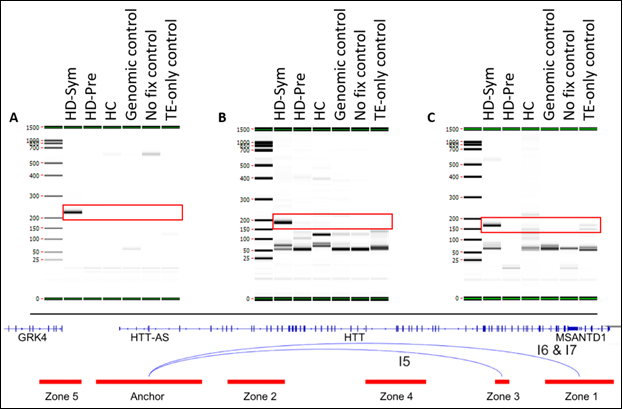
Figure 6: Summary of conditional interactions-Huntington’s Disease Positive.

Figure 7: Conditional interactions observed in individual HD-Sym samples.
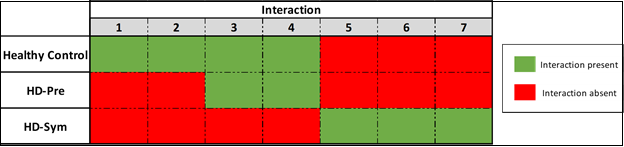
Figure 8: Model for epigenetic changes driving the symptomatic progression of Huntington’s disease.
Discussion
Problem
Statement and Results Summary
While it is well-known that individuals with greater than 39 CAG-repeats will get HD, the clinical onset of disease varies widely amongst individual patients and the factors that influence when the disease manifests clinically are less well characterized. Here we used EpiSwitch®, an industrial platform for assessing chromatin architecture, to evaluate the epigenomic landscape of the HTT locus in HD patients and healthy, unaffected controls. We identified a set of seven interactions that when taken together as a CCS, could differentiate HD from unaffected controls and more importantly, could differentiate between presymptomatic and symptomatic HD patients. One of these interactions, specific for symptomatic HD, contains a SNP (rs362331) shown to be associated with a predisposing disease haplogroup. When taken together, these results provide an initial indication that a simple, non-invasive blood-based test evaluating a CCS deserves further study and validation as a surrogate biomarker for assessing disease progression in HD.
Biological
Relevance
While
it is known that the poly-Q repeat tract expansion and production of mHTT are
the underlying causes of HD, the molecular events leading to the development of
clinical symptoms are less well characterized. Several studies have looked at
SNPs within the HTT locus as a potential contributor to disease onset. One
recent SNP genotyping study of HD patients identified ~41 SNPs heterozygous in
at least 30% of the patients, including the rs362331 C/T SNP in exon 50 of the
HTT gene [43]. Perhaps more biologically relevant is that when the rs362331 SNP
is allele-selectively knocked down using anti-sense oligonucleotides, siRNAs or
miRNA, a dramatic reduction in the levels of mHTT protein is achieved both in
vitro and in vivo suggesting that this SNP and its surrounding genomic
landscape play an important role in regulating mHTT levels [44-46]. In this
study, we observed a chromosome conformation (I5) that was present only in
HD-Sym patients and absent in HD-Asy and HCs that overlapped with the rs362331
SNP. While requiring further study, this observation raises the interesting
possibility that the production of neurotoxic mHTT in patients that have
increased poly-Q tracts and a genetic predisposition to the early development
of HD by the presence of the rs362331 SNP may be regulated at the level of
higher order chromatin structure. Another outstanding question in HD is how the
disease is inherited in cases where neither parent has received a diagnosis.
The
two main prevailing hypotheses posit that 1) the carrier parent could have
passed away from another factor before the onset of the disease and 2)
“unstable” CAG repeat tracts expand with each generation. A third possibility
also exists, in that at mid-range (35-50) repeats, individuals could be
carriers without manifestation of the disease, but their progeny might be
unable to compensate for the genetic defect through undefined mechanisms and
will develop the disease. The HD patients evaluated in this study all had CAG
repeats in this mid-range, raising the possibility that potential compensatory
mechanisms in disease development may be mediated through differences in
genomic architecture.
Clinical
Relevance
HD
is unique in that there exists a simple test to definitively diagnose the
disease, HTT gene sequencing and measurement of CAG repeat number. For clinical
care and clinical trials, there are also several tests to measure disease
severity, such as the Unified Huntington’s Disease Rating Scale (UHDRS), the
Shoulson-Fahn Scale, and the Mini-Mental State Examination (MMSE). While these
assessments measure different elements of an HD patients physical and mental
well-being as a surrogate for disease severity, they are all subjective in
nature and most are not specific for HD. What is missing are concrete molecular
tools to monitor disease progression.
As
of the time of this writing, there are 22 therapeutic agents for treating HD in
different stages of preclinical and clinical development, half of which are in
Phase 2 or Phase 3. Once further validated, the CCS reported here could be used
in clinical trials as a surrogate outcome biomarker to assess the therapeutic
efficacy of the drug in question. In addition to monitoring a symptomatic
patient’s response to a particular therapy in clinical trials, another
advantage of the approach described here lies in the information that can be obtained
for presymptomatic patients. For most HD patients, the presymptomatic period
can last decades. Five of the seven (i3-i7) interactions identified here
clearly separate presymptomatic HD patients from symptomatic ones, and when
further validated could serve as an “early warning” indicator test for the
onset of HD symptoms in presymptomatic carriers.
Strengths
and Limitations
This
study gives first evidence of detectable conditional differences in chromatin
architecture specific for the manifestation of HD and correlated with known
disease haplotypes. The major strength of this study lies in the unique
approach, which is based on the latest developments in understanding the
regulatory role of genomic architecture. While there have been several historical
studies in HD aimed at developing disease progression biomarkers based on
clinical, imaging and molecular measures [47], to the best of our knowledge
this is the first time that the assessment of higher order chromatin structures
in a clinically accessible biofluid has been applied in HD. Already
successfully applied in another neurodegenerative condition, amyotrophic
lateral sclerosis, as well as other non-neurological conditions such as
melanoma, diffuse large B-cell lymphoma, chronic myelogenous leukemia, thyroid
cancer, prostate cancer, lung cancer, breast cancer, and rheumatoid arthritis,
the results presented here further validate the use of regulatory conditional
CCS as disease-related biomarkers [25-33]. Of note, these previous studies
using CCS as biomarkers of disease have been done on larger sample sizes. A
notable limitation of this study was the relatively small sample size, a
limitation partially imposed by the rare nature of HD. While the data presented
here offer a novel insight into the clinical progression of HD, this study was
intended to be a proof-of-concept and not powered for statistical significance.
A follow-up study using a larger patient cohort will be required for validation
of these initial results.
Conclusions
Here
we provide the first evidence that chromatin architecture at the HTT locus is
systemically altered in patients with HD and show conditional differences
between clinical stages. Our results strongly suggest that the further
development of a non-invasive blood test based evaluating Chromosome
Conformation Signatures (CCS) would offer a novel molecular approach to
assessing likely disease severity and progression in HD.
Acknowledgements
The
authors would like to thank members of OBD Reference Facility for direct operational
support and taking part in processing of clinical samples.
References
- Ross CA, Aylward EH,
Wild EJ, Langbehn DR, Long JD, et al.
Huntington disease: Natural history, biomarkers and prospects for therapeutics
(2014) Nature Revi Neurol 10: 204-216. https://doi.org/10.1038/nrneurol.2014.24
- Rawlins MD, Wexler NS,
Wexler AR, Tabrizi SJ, Doughlas I, et al. The prevalence of huntington’s
disease. Neuroepidemiology (2016) Neuroepidemiol 46: 144-153. https://doi.org/10.1159/000443738
- MacDonald ME, Ambrose
CM, Duyao MP, Myers RH, Lin C, et al. A novel gene containing a trinucleotide
repeat that is expanded and unstable on Huntington’s disease chromosomes (1993)
Cell 72: 971-983. https://doi.org/10.1016/0092-8674(93)90585-E
- Gusella JF, Wexler NS,
Conneally M, Naylor SL, Anderson MA, et al. A polymorphic DNA marker
genetically linked to Huntington’s disease (1983) Nature 306: 234-238. https://doi.org/10.1038/306234a0
- Myers RH. Huntington’s
disease genetics (2004) NeuroRx 1: 255-262. https://doi.org/10.1602/neurorx.1.2.255
- Chaganti SS, McCusker
EA and Loy CT. What do we know about late onset Huntington’s disease? (2017) J
Huntington’s Dis 6: 95-103. https://doi.org/10.3233/jhd-170247
- Duff K, Beglinger LJ
and Paulsen JS. ‘Pre-symptomatic’ Huntington’s disease (2008) Handbook Clinical
Neurol 89: 589-598. https://doi.org/10.1016/s0072-9752(07)01255-9
- Valor LM.
Transcription, epigenetics and ameliorative strategies in Huntington’s disease:
a Genome-wide perspective (2014) Molecular Neurobiol 51: 406-423. https://doi.org/10.1007/s12035-014-8715-8
- Cha JHJ. Transcriptional
signatures in Huntington’s disease (2007) Prog Neurobiol 83: 228-248. https://doi.org/10.1016/j.pneurobio.2007.03.004
- Santiago JA and
Potashkin JA. A network approach to clinical intervention in neurodegenerative
diseases (2014) Tre Mol Med 20: 694-703. https://doi.org/10.1016/j.molmed.2014.10.002
- Nayak A, Salt G, Verma
SK and Kishore U. Proteomics approach to identify biomarkers in
neurodegenerative diseases (2015) Int Rev Neurobiol 121: 59-86. https://doi.org/10.1016/bs.irn.2015.05.003
- Moss DJH, Pardiñas FA,
Langbehn D, Lo K, Leavitt RB, et al. Identification of genetic variants
associated with Huntington’s disease progression: a genome-wide association
study (2017) Lancet Neurol 16: 701-711. https://doi.org/10.1016/S1474-4422(17)30161-8
- Graham SF, Pan X,
Yilmaz A, Macias S, Robinson A, et al. Targeted biochemical profiling of brain
from Huntington’s disease patients reveals novel metabolic pathways of interest
(2018) Biochim Biophys Acta Mol Basis Dis 1864: 2430-2437 https://doi.org/10.1016/j.bbadis.2018.04.012
- Francelle L, Lotz C,
Outeiro T, Brouillet E and Merienne K. Contribution of Neuroepigenetics to
Huntington’s Disease (2017) Front Hum Neurosci. https://doi.org/10.3389/fnhum.2017.00017
- Achour M, Le Gras S,
Keime C, Parmentier F, Lejeune FX, et al. Neuronal identity genes regulated by
super-enhancers are preferentially down-regulated in the striatum of
Huntington’s disease mice (2015) Hum Mol 24: 3481-3496. https://doi.org/10.1093/hmg/ddv099
- Valor LM and Guiretti
D. What’s wrong with epigenetics in Huntington’s disease? (2014) Neuropharmacol
80: 103-114. https://doi.org/10.1016/j.neuropharm.2013.10.025
- Glajch KE and
Sadri-Vakili G. Epigenetic mechanisms involved in Huntington’s disease
pathogenesis (2015) J Huntington’s Dis 4: 1-15. https://doi.org/10.3233/JHD-140134
- Valor LM, Guiretti D,
Lopez-Atalaya JP and Barco A. Genomic landscape of transcriptional and
epigenetic dysregulation in early onset polyglutamine disease (2013) J Neurosci
33: 10471-10482. https://doi.org/10.1523/JNEUROSCI.0670-13.2013
- McFarland KN, Das S,
Sun TT, Leyfer D, Xia E, et al. Genome-wide histone acetylation is altered in a
transgenic mouse model of Huntington’s disease (2012) PLoS One 27. https://doi.org/10.1371/journal.pone.0041423
- Tordini F, Aldinucci M,
Milanesi L, Li P and Merelli I. The genome conformation as an integrator of
multi-omic data: The example of damage spreading in cancer (2016) Front Genet https://doi.org/10.3389/fgene.2016.00194
- Tordini F, Aldinucci M,
Milanesi L, Lio P and Merelli I. The Genome Conformation As an Integrator of
Multi-Omic Data : The Example of Damage Spreading in Cancer (2016) Frontiers 7.
https://doi.org/10.3389/fgene.2016.00194
- Dekker J, Rippe K,
Dekker M and Kleckner N. Capturing chromosome conformation (2002) Science 295:
1306-1311. https://doi.org/10.1126/science.1067799
- Bastonini E et al.
Chromatin barcodes as biomarkers for melanoma (2014) Pigment Cell Melanoma Res
27: 788-800. https://doi.org/10.1111/pcmr.12258
- Crutchley JL, Wang XQD,
Ferraiuolo MA and Dostie J. Chromatin conformation signatures: ideal human
disease biomarkers? Biomark (2010) Med 4: 611-629. https://doi.org/10.2217/bmm.10.68
- Jakub JW, Travis EG,
Philip J, Ewan H, Mark P, Aroul R, et al. A pilot study of chromosomal
aberrations and epigenetic changes in peripheral blood samples to identify patients
with melanoma (2015) Melanoma Res 25: 406-411. https://doi.org/10.1097/CMR.0000000000000182
- Mukhopadhyay S,
Ramadass AS, Akoulitchev A and Gordon S. Formation of distinct chromatin conformation
signatures epigenetically regulate macrophage activation (2014) Int
Immunopharmacol 18: 7-11. https://doi.org/10.1016/j.intimp.2013.10.024
- Carini C, Hunter E,
Ramadass AS, Green J, Alexandre A, et al. Chromosome conformation signatures
define predictive markers of inadequate response to methotrexate in early
rheumatoid arthritis (2018) J Transl Med 18. https://doi.org/10.1186/s12967-018-1387-9
- Cao F, Fang Y, Tan HK,
Goh Y, Choy JYH, et al. Super-enhancers and broad h3k4me3 domains form complex
gene regulatory circuits involving chromatin interactions (2017) Sci Rep 2186. https://doi.org/10.1038/s41598-017-02257-3
- Grand FH, Bird C,
Corfield E, Dezfouli M, Warren E, et al. Chromatin conformation signatures
associated with epigenetic deregulation of the FIP1L1 and PDGFRA genes (2016)
Blood 128: 1525. https://doi.org/10.1182/blood.V128.22.1525.1525
- Yan H, Hunter E,
Alexandre A, Park P, David WJ, et al. Epigenetic chromatin conformation changes
in peripheral blood can detect thyroid cancer (2019) Surg 165: 44-49. https://doi.org/10.1016/j.surg.2018.05.081
- Hunter E, McCord R,
Ramadass SA, Green J, Westra WJ, et al. Comparative molecular cell-of-origin
classification of diffuse large B-cell lymphoma based on liquid and tissue
biopsies (2020) Transl Med Commun 5. https://doi.org/10.1186/s41231-020-00054-1
- Alshaker H, Mills R,
Hunter E, Salter M, Ramadass A, et al. Chromatin conformation changes in
peripheral blood can detect prostate cancer and stratify disease risk groups
(2021) J Transl Med https://doi.org/10.1186/s12967-021-02710-y
- Shah P, Potluri S,
Zhang S, Dezfouli M, Back J, et al. Development and validation of baseline
predictive biomarkers for response to avelumab in second-line (2L) non-small
cell lung cancer (NSCLC) using EpiSwitchTM epigenetic profiling (2019) J
Immunother Cancer 7.
- Salter M, Corfield E,
Ramadass A, Grand F, Green J, et al. Initial Identification of a Blood-Based
Chromosome Conformation Signature for Aiding in the Diagnosis of Amyotrophic
Lateral Sclerosis (2018) EBio Medicine 33: 169-184. https://doi.org/10.1016/j.ebiom.2018.06.015
- Poesen K. The
Chromosomal Conformation Signature: A New Kid on the Block in ALS Biomarker
Research? (2018) EBio Medicine 33: 6-7. https://doi.org/10.1016/j.ebiom.2018.07.003
- Barrett T, Wilhite ES,
Ledoux P, Evangelista C, Kim FI, et al. NCBI GEO: Archive for functional
genomics data sets-Update (2013) Nucleic Acids Res 41. https://doi.org/10.1093/nar/gks1193
- Dong X, Tsuji J,
Labadorf A, Roussos P, Chen FJ, et al. The role of H3K4me3 in transcriptional
regulation is altered in Huntington’s disease (2015) PLoS One 2015. https://doi.org/10.1371/journal.pone.0144398
- The HD iPSC Consortium,
Lim R and Salazar L. Developmental alterations in Huntington’s disease neural
cells and pharmacological rescue in cells and mice (2017) Nat Neurosci 20:
648-660. https://doi.org/10.1038/nn.4532
- Robinson JT,
Thorvaldsdóttir H, Winckler W, Guttman M, Lander SE, et al. Integrative
genomics viewer (2011) Nature Biotechnology 29: 24-26. https://doi.org/10.1038/nbt.1754
- James WJ, Travis EG, Philip
J, Ewan H, Mark P, et al. A pilot study of chromosomal aberrations and
epigenetic changes in peripheral blood samples to identify patients with
melanoma (2015) Melanoma Res 25: 406-411. https://doi.org/10.1097/CMR.0000000000000182
- Carini C, Hunter E,
Ramadass SA, Green J, Akoulitchev A, et al. Chromosome conformation signatures
define predictive markers of inadequate response to methotrexate in early
rheumatoid arthritis (2018) J Transl Med 16. https://doi.org/10.1186/s12967-018-1387-9
- Salter M, et al.
Epigenetic signatures and early detection of neurodegenerative diseases. in The
Lancet Neurology Conference (The Lancet Neurology Conference, 2016).
- Carroll JB, Warby CS,
Southwell LA, Doty NC, Greenlee S, et al. Potent and selective antisense
oligonucleotides targeting single-nucleotide polymorphisms in the huntington
disease gene/allele-specific silencing of mutant huntingtin (2011) Mol Ther 19:
2178-2185. https://doi.org/10.1038/mt.2011.201
- Miller JRC, Pfister LE,
Liu W, Andre R, Trägeret U, et al. Allele-Selective Suppression of Mutant
Huntingtin in Primary Human Blood Cells (2017) Sci Rep 7: 46740. https://doi.org/10.1038/srep46740
- Pfister EL, Kennington
L, Straubhaar J, Vonsattel JP, Zamoreet DP, et al. Five siRNAs Targeting Three
SNPs May Provide Therapy for Three-Quarters of Huntington’s Disease Patients
(2009) Curr Biol 19: 774-778. https://doi.org/10.1016/j.cub.2009.03.030
- Miniarikova J, Zanella I, Huseinovic A, van Deventer JS, Petry H, et al. Design, Characterization, and Lead Selection of Therapeutic miRNAs Targeting Huntingtin for Development of Gene Therapy for Huntington’s Disease (2016) Mol Ther-Nucleic Acids 5. https://doi.org/10.1038/mtna.2016.7
- Killoran A and Biglan
K. Biomarkers for Huntington’s disease: A brief overview (2016) J Rare Dis Res
Treat.
*Corresponding author:
Alexandre Akoulitchev, Oxford BioDynamics Plc, First Floor, Building 7600 C, The Quorum, Alec Issigonis Way, Oxford Business Park North, OX4 2JZ, United Kingdom, Tel: +44 7796 666 405, E-mail: alexandre.akoulitchev@oxfordbiodynamics.com
Citation:
Salter M, Powell R, Back J, Grand F, Koutsothanasi C, et al.
Genomic architecture differences at the HTT locus associated with symptomatic
and pre-symptomatic cases of Huntington’s disease in a pilot study
(2021) Edelweiss Psyi OpenAccess 5: 1-6.
Keywords
Huntington’s disease, Epigenetics, Chromosome
conformation signature, Chromatin architecture.


 PDF
PDF