Introduction
In recent years, the search for inexpensive and eco-friendly synthesis routes has increased significantly. Nanotechnology and biotechnology have established themselves as a major ally in building green technologies for effective, stable, and non-toxic nanomaterial synthesis. [1-3]. Thus, the principles of green bio nanotechnology are associated with waste prevention, maximizing atom economy, and less use of precursors with less hazardous synthesis routes and the use of safe chemicals with low toxicity. Reaction conditions are also advantageous due to safe methodologies and increased energy efficiency.
The association of these principles with the use of renewable raw materials that avoid chemical derivation, such as the plant environment, is preferable because it does not generate harmful by-products to the environment or the health of living beings. Besides, the system activation energy decreases with catalysts rather than stoichiometric reagents [4,5]. For green synthesis, a substance or set of substances produced by nature must be able to form, through a physicochemical process, molecular entities of compositions, morphologies, sizes, and surface charges synergistically linked to the dispersive medium compounds and elementarily of the precursor material used for the process [6].
Given this, the set of substances that will be used in the process of formation of nanomaterials will define the efficiency of the synthesis process, its stabilization, and possible technical-scientific applications. Therefore, seasonality is intrinsic to the green synthesis of nanomaterials by setting variations concerning the molecular concentrations present in the same extractive route throughout the year.
Green Synthesis of Nanoparticles
Green synthesis has excellent value in nanotechnology. One example is the synthesis of metal nanoparticles using surface reducing and Stabilizing agents generated from natural sources such as fruits, fruit peel, seeds, leaves, root or other non-toxic sources [7-11]. The stabilizing agents, in this case, are biological substances such as phenolic compounds that favor the green synthesis route [12]. The properties of nanoparticles will depend mainly on size and morphology, so controlling the parameters of the routes is very important. The nanoparticle nucleation begins with a spherical shape. From a critical radius, the morphology will depend intrinsically on the feature of the dispersive medium, mainly due to the supramolecular interaction of the molecules of dispersive medium with the crystalline planes of metallic nanoparticles.
If it is the same for all planes, the tendency is for nanoparticles to remain spherical. If different, nanoparticle shapes may vary with preferential growth in planes where affinity is lower with the medium [13]. In general, the molecules present in the dispersive medium will also influence the kinetics of chemical reactions, depending on the concentration and chemical nature. The higher the concentration of bioactive molecules in a dispersive medium, the smaller the particle growth [14]. Thus, organic growth conditions, seasonality, and extraction methods can influence the concentrations of antioxidant chemical species and generate nanostructures with varied characteristics directly related to bioavailable molecules in the green synthesis route of nanoparticles [15,16].
Nanoparticle Biosynthesis
In state-of-the-art, Nanoparticles (NPs) are particles that have different properties and applications when compared to their micro or macro (bulk) scale. These peculiar quantum phenomena usually occur in 0D nanomaterials when their size is less than 100 nanometers [17]. The biosynthesis of metallic nanoparticles occurs through the reduction of an ion to the fundamental state atom through its interaction with the dispersive medium. The biosynthesis begins a process of growth and nucleation, where the atoms unite forming nanoparticles. Stabilization of growth and preventing agglomeration of other nucleating particles is the responsibility of the dispersive medium [18]. Finally, a colloidal matrix is established in which the extractive medium inserts its physicochemical characteristics and active principles around the nanoparticles, modulating their reactivity, morphology, size and surface charge. Therefore, the control of the concentration and molecules are present in the dispersive medium is essential to obtain protocols that overcome seasonality. Green synthesis can use as a dispersive medium plant extract, microorganism cultures (or only their products), and animal products as alternative routes to traditional methods that, in synergy with nanoparticles, allow a wide diversity of bionanotechnological applications [19-21].
Seasonal and Botanical Factors Associated with Environmental Pollution
Environmental pollution can also interfere with plant biology. Thus, the action of atmospheric pollutants is more severe in the leaves, with a high degree of leaf injury related to the resistance of the plant to contaminants [22]. Plants exposed to acid rain, for example, have the youngest Trifolium near the apex with rough morphology. The shortening of the internodes is observed, and the ribs of the older leaves are reddish. These symptoms may have been the result of mesophilic cell hyperplasia or hypertrophy [23, 24]. There is also early leaf fall, as described in the literature, as a typical compensation mechanism in plants exposed to sulfur dioxide, a substance present in acid rain [24]. There is also a decrease in the number of glandular trichomes. This event influences the production of the main antioxidant compounds associated with green nanoparticle synthesis. It is important to emphasize that studies are needed regarding the possible impacts of pesticides used in commercial plantations that may also affect the plant to influence the efficiency of the synthesis route of nanoparticles. Thus, the plant tends to increase the concentration of defense substances against reactive species harmful to their tissues, such as some phenolic compounds, when exposed to stress [25].
Botanical and Physicochemical Characteristics associated with Seasonality
During plant formation, plants develop various chemical species and cellular structures, as well as peculiar organs such as roots, flowers, fruits, leaves, among others. Thus, several factors influence the formation of these organs, since the availability of water, sunlight, soil microorganisms as well as specific temperatures determine the development, death or inactivation of fundamental structures for plant growth and survival given that plants always seek to adapt to energetically favorable conditions [26-28].
Leaves are the most bioavailable structures present in a plant, and several structural and metabolic chemical species make up the biology of this plant organ. Then, the abundant presence of polymers that have strong in nature intra, inter, and supramolecular interactions such as lignin and cellulose preserve the properties of the leaves. Then extractions occur in a more standardized and controlled manner in the face of seasonality and climate adversity. As an example, the leaves of Psidium guajava Linn. (Guava tree) have high concentrations of lignin-cellulosic structures that preserve extractive patterns throughout the year [29,30]. As with guava leaves, the use of an extract from Morus nigra L leaves (blackberry) leads to successful synthesis routes for nanoparticle formation. However, over a period, which coincides with winter, nanoparticle synthesis is not efficient, and this fact may be associated with seasonal characteristics because, during this period, there is a reduction in leaf size and morphological changes in themselves [31].
Moreover, during the synthesis of the extract, the variation in coloration from intense golden yellow to light yellow is observed. This observation suggests that basal molecules for the formation and stabilization of nanoparticles are being affected due to seasonality. Additionally, regarding the seasonality of the fruit of the genus Euterpe Oleracea (açaí), it is possible to mention the differences presented between the fruits in the green ripening stage and the ripe fruit, where, according to several studies, there is a discrepancy in the measured values of phenolic compounds and anthocyanins for these fruits [32-34].
In our recent studies, it can be possible to observe that there is an essential difference between the ripe and immature fruit, principally about the intensity of surface plasmon resonance. This date expresses the efficiency of synthesis associated with the efficiency dispersive medium associated with the dispersion of nanoparticles. In the literature, there is an association of phenolic compounds in fruits at different stages of ripeness, and it can be possible to observe that the content of the major phenolic constituents decreases from green to mature stages. In general, it can be possible to observe that in mature to senescent fruit stages, there is an increase of antioxidant compounds, but in some cases, the opposite occurs, i.e., the decrease of phenolic compounds. Thus, it is possible to observe that mainly phenolic and flavonoid acids are correlated with ripening stages depending on the fruit so, there is a significant variation in the concentration of antioxidant compounds that are fundamental for the adjustments in the green synthesis routes [35,36].
This quantitative difference of compounds in fruit composition is directly related to the synthesis of Silver Nanoparticles (AgNPs). In the case of root, one case studied was related to Beta vulgaris (beetroot), because its plant extract showed stability in the synthesis of AgNPs throughout the year, showing no changes associated with seasonality. This invariability can be attributed to the stability of its secondary metabolites and genetic information in crop reproduction. It can be possible to found in the literature that beetroot have karyotype, and the number of chromosomes preserved [37]. Rainier or drier times of the year, as well as the four seasons, directly influence the maturation of plant structures; In addition, various environmental and genetic conditions can alter the range of substances present in plants such as the composition of the land on which the plant is grown, phylogeny, as well as the biodiversity of the plant species. [38]. Therefore, seasonal factors such as soil pH, temperature, humidity, climate, flora, region, as well as the ecosystem in general, may influence the chemical composition and accumulation of secondary metabolites present in plant species and, in addition, these seasonal conditions are related to formations, development, and changes in the basal structures present in plants [39,40].
Seasonality and Organic Compounds in Synergy with Green Nanoparticle Synthesis
Plants have specialized structures capable of secreting essential oils from the accumulation of secondary metabolites such as glandular trichomes, which are specialized structures present in the leaf epidermis capable of secreting substances such as flavonoids and phenolic compounds in general [41]. These organic compounds make up the group of polyphenols that are associated with various antioxidants, antimicrobial, antiviral, antimutagenic activities, among others [42]. Also, flavonoids and phenolic compounds may be present in the plant as well as animal raw materials found in bee products such as propolis. Thus, propolis is a natural resin produced exclusively by the work done by bees, and, in this sense, it has been shown in the literature that the biological characteristics of propolis vary significantly according to the time of year in which it was collected [43].
Therefore, Figure 1 demonstrates the influence of seasonality on organic materials of plant and animal origin in the formation of nanoparticles according to a sustainable bias characteristic of green synthesis.
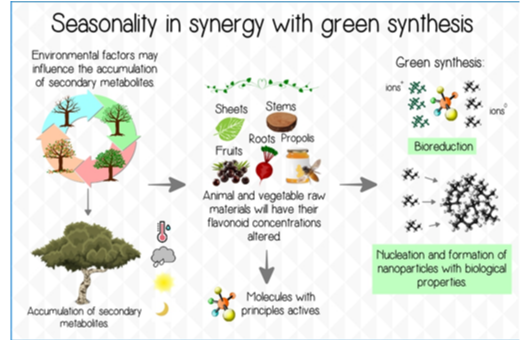
Figure 1: The representative scheme demonstrates the relationship of molecules with active principles from plant and animal products such as roots, leaves, fruits, stems and propolis, in synergy with the influence of environmental factors and synthesis pathway parameters on the formation of nanoparticles.
In this context, seasonality can significantly change the chemical composition of plant and animal products. Phenolic compounds such as bacarina were present in high concentrations of propolis obtained during autumn and winter compared to other seasons, and the literature demonstrates that seasonality influences the concentration and activity of antioxidant chemical species that are extremely important for nanoparticle synthesis [43]. Plants have several chemicals, morphological, and anatomical mechanisms to adapt to variations that occur throughout the seasons. The leaves play an essential role in these adaptations because they can deactivate photosynthesis, altering the chemical composition of the cell wall and carbohydrates in cells, as well as degrading chlorophyll. Some plants present different responses to water stress in rainy and dry seasons, being able to tolerate water loss through mechanisms that occurred only in the dry season, such as leaf cover by compounds that protect the plant from an excess of radiation, as well as the accumulation of sugars such as sucrose, raffinose, and arabinose [44].
It is also important to highlight that in stressful situations, the plants tend to increase the production of antioxidant compounds to avoid damage caused by reactive species. These variations may influence the synthesis of metallic nanoparticles, as these compounds are fundamental in reducing the precursor ion and stabilizing the colloidal matrix. Then, it is crucial to establish between the natural extracts and the efficient synthesis routes, with parameters that produce nanoparticles with smaller cluster formation. Although several studies have been performed with different extractive routes from different natural origins, it is not known precisely, which compound or set of compounds would be leading to a successful green synthesis of nanoparticles for bionanotechnological applications [45].
Therefore, the question arises: Can green synthesis of nanoparticles be efficient all year long? It is well known that some plant components certainly have a favorable relationship to the green synthesis of nanoparticles, such as phenolic compounds, anthocyanins, flavonoids, tannins, carbohydrates, and proteins. However, even with the wide variety of composition between the different natural extracts, studies are also needed regarding the variation of green synthesis efficiency throughout the year. The relationship between the morph anatomical and phytochemical characteristics associated with the features of the microenvironment in which the dispersive medium is extracted, besides the influences of the regions ecosystem, are aspects of great importance related to the green nanoparticle routes. Additionally, it is also relevant to note that the variation in metabolites related to plant organ growth and development directly affects the use of extracts for biosynthetic pathways due to changes in chemical structures that occur in synergy with seasonality.
Final Considerations
Given all that has been addressed, we are inviting the scientific community to evaluate how green synthesis has efficiency throughout the year. According to the peculiar characteristics of natural extracts associated with seasonality, it should be taken into consideration that physicochemical characterizations should be performed. From there, the substances responsible for the reduction and stabilization of metallic nanoparticles produced by green synthesis must have a repetitive and scientifically stable protocol for the dispersion and stabilization of these nanostructures, guaranteeing, in fact, Nano technological products.
References
1. Ram Prasad. Synthesis of silver nanoparticles in photosynthetic plants (2014) J Nano 1-8. https://doi.org/10.1155/2014/963961
2. Raveendran P, Fu J and Wallen SL. A simple and green method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles (2006) Green Chem 8: 34-38. https://doi.org/10.1039/B512540E
3. Keat CL, Aziz A, Eid AM and elmarzugi AN. Biosynthesis of nanoparticles and silver nanoparticles (2015) Bioresour. Bioprocess 2: 47. https://doi.org/10.1186/s40643-015-0076-2
4. Cauerhff A and Castro G. Bionanoparticles, a green nanochemistry approach (2013) Electron J Biotechnol 16:717-3458. https://doi.org/10.2225/vol16-issue3-fulltext-3
5. Akhtar BS, Panwar J and Yun YS. Biogenic synthesis of metallic nanoparticles by plant extracts (2013) ACS Sustain Chem Eng 1: 591-602. https://doi.org/10.1021/sc300118u
6. Iravani S. Green synthesis of metal nanoparticles using plants (2011) Green Chemistry 13: 2638–2650. https://doi.org/10.1039/C1GC15386B
7. B Kumar, K Smita, L Cumbal, A Debut. Green synthesis of silver nanoparticles using andean blackberry fruit extract (2017) Saudi J Bio Sci 24:45-50. https://doi.org/10.1016/j.sjbs.2015.09.006
8. Das G, Patra JK, Debnath T, Ansari A and Shin H-S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.) (2019) 14. https://doi.org/10.1371/journal.pone.0220950
9. Afifa Qidwai, Rajesh Kumar and Anupam Dikshit. Green synthesis of silver nanoparticles by seed of Phoenix sylvestris L. and their role in the management of cosmetics embarrassment (2018) Green Chemistry Letters and Reviews, 11: 176-188. https://doi.org/10.1080/17518253.2018.1445301
10. M. Anandan, G Poorani, P Boomi, K Varunkumar and K Anand, et al. Green synthesis of anisotropic silver nanoparticles from the aqueous leaf extract of dodonaea viscosa with their antibacterial and anticancer activities (2019) Process Biochem 80: 80-88. https://doi.org/10.1016/j.procbio.2019.02.014
11. Tessy John, Kokila A Parmar and Paras Tak, Biosynthesis and characterization of silver nanoparticles from Tinospora cordifolia root extract (2019) J Nanosci. Tech. 5: 622-626. https://doi.org/10.30799/jnst.211.19050112
12. Kakakhel, Ahmad S, Sidra M, Zeb N and Ullah Asad et al. (2019) R E V I E W Green nanotechnology: a review on green synthesis of silver nanoparticles an ecofriendly approach. International J Nanomed 14: 5087–5107. https://doi.org/10.2147/ijn.s200254
13. Sukdeb P, Tak Y K and Song J M (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? a study of the gram-negative bacterium escherichia coli. Applied and environmental microbiology. 7: 1712-1720. https://doi.org/10.1128/aem.02218-06
14. Christian, Pfeiffer, Rehbock, Christoph and Hühn et al. Interaction of colloidal nanoparticles with their local environment: the (ionic) nanoenvironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles (2014) J Royal Society 11. https://doi.org/10.1098/rsif.2013.0931
15. Anupam Z, Onur B, Sudip S, Mandal K A and Yilmaz M (2018) Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Advances 9: 2673-2702. https://doi.org/10.1039/c8ra08982e
16. Anjum S, Abbasi, Bilal, Shinwari and Zabta. plant-mediated green synthesis of silver nanoparticles for biomedical applications: challenges and opportunities (2016) Pakistan J Bot 48: 1731-1760. https://doi.org/10.1016/B978-0-323-41533-0.00006-4
17. Tiwari JN, Tiwari RN and Kim KS. Zero-dimensional, one-dimensional, two-dimensional, and three-dimensional nanostructured materials for advanced electrochemical energy devices (2012) Progress in Materials Sci 57: 724-803. https://doi.org/10.1016/B978-0-323-41533-0.00006-4
18. Thakkar KN, Mhatre, SS and Parikh RY. Biological synthesis of metallic nanoparticles Nanomedicine: Nanotechnology (2010) Biology and Med 6: 257-262. https://doi.org/10.1016/j.nano.2009.07.002
19. Bumbudsanpharoke N and Ko S. Nano-food packaging: an overview of market, migration research, and safety regulations (2015) J Food Sci 80: 910-923. https://doi.org/110.1111/1750-3841.12861
20. Cauerhff A, Castro G. Bionanoparticles, a green nanochemistry approach (2013) Electron J Biotechn 16: 717-3458. https://doi.org/10.2225/vol16-issue3-fulltext-3
21. Backx BP, Pedrosa BR, Delazare T, Damasceno FRDC, Santos OALD et al. Green synthesis of silver nanoparticles: A study of the dispersive efficiency and antimicrobial potential of the extracts of Plinia cauliflora for application in smart textiles materials for healthcare (2018) J Nanomater 6. https://doi.org/10.4172/2324-8777.1000236
22. Silva LC, Oliva MA and Azevedo AA. Micromorphological and anatomical alterations caused by simulated acid rain in resting plants: Eugenia uniflora and Clusia Hilariana. Water, Air, and Soil Pollution (2005) 158: 129-143. https://doi.org/10.1007/s11270-005-0941-2
23. Sant’anna-Santos BF, Silva LC, Azevedo AA and Aguiar R. Effects of simulated acid rain on leaf anatomy and micromorphology of Genipa Americana L. (Rubiaceae) (2006) Brazilian Archives of Biology and Technology 49: 313-321. http://dx.doi.org/10.1590/S1516-89132006000300017
24. Szabo AV, Domingos M, Rinaldi MCS and Delitti WBC. Acúmulo de enxofre e suas relações com alterações no crescimento de plantas jovens de Tibouchina pulchra Cogn. (Melastomataceae) expostas nas proximidades do pólo industrial de Cubatão, SP (2003) Revista Brasileira de Botânica, 26: 379-390. http://dx.doi.org/10.1590/S0100-84042003000300011
25. Faller ALK and Fialho E. Polyphenol content and antioxidant capacity in organic and conventional plant foods (2010) J Food Composition and Analysis, Horticulture, Biodiversity, and Nutrition 23: 561–568. https://doi.org/10.1016/j.jfca.2010.01.003
26. Crocker W. Growth of Plants (1948) Soil Science 66: 79.
27. Plenchette C, Fortin JA and Furlan V. Growth responses of several plant species to mycorrhizae in a soil of moderate P-fertility (1983) Plant and Soil 70: 199–209. https://doi.org/10.1007/BF02374780
28. Sparks TH, Huber K and Croxton PJ. Plant development scores from fixed-date photographs: the influence of weather variables and recorder experience (2006) International J Biometeorology 50: 275-279. https://doi.org/10.1007/s00484-005-0022-7
29. Camarena-Tello JC, Rocha-Guzmán NE, Gallegos-Infante JA, González-Laredo RF and Pedraza-Bucio FE et al. Chemical composition of biomass generated in the guava tree pruning (2015) EXCLI J 14: 204-212. http://dx.doi.org/10.17179/excli2014-467
30. Duarte MDR and De Paula FM. Morfodiagnose de Psidium guajava L., MYRTACEAE (2005) Visão Acadêmica 6. http://dx.doi.org/10.5380/acd.v6i2.6112
31. Biasiolo M, Canal MTD and Tornadore N. Micromorphological characterization of tem mulberry cultivars (Morus spp) (2004) Econ Bot 58: 639-646. https://doi.org/10.1663/0013-0001(2004)058[0639:mcotmc]2.0.co;2
32. Blank, DE, Justen D, Fraga S, Peixoto CR and de Moura NF et al. Chemical Composition and Antioxidant Activity of Bunchosia glandulifera Fruit at Different Ripening Stages (2018) Scientific Research Publishing 9: 1147-1159. https://doi.org/10.4236/fns.2018.910083
33. Borges GDSC, Vieira FGK, Copetti C, Gonzaga LV and Zambiazi RC et al. Chemical characterization, bioactive compounds, and antioxidant capacity of jussara (Euterpe edulis) fruit from the Atlantic Forest in southern Brazil (2011) Food Research International. Elsevier 44: 2128-2133. https://doi.org/10.1016/j.foodres.2010.12.006
34. Backx BP and Santana JCS. Green Synthesis of Polymer Blend impregnated with silver nanoparticles in Euterpe Oleracea dispersive medium (2018) International J Green and Herbal Chemistry 7: 424-429. https://doi.org/10.24214/IJGHC/GC/7/2/42429
35. Silva, Marques da K, Zielinski, Ferreira A A and Benvenutti et al. Efeito do amadurecimento de frutos em compostos bioativos e capacidade antioxidante de bebidas de maçã. Ciência e Tecnologia de Alimentos (2019) Food Sci. Technol 39: 294-300. https://dx.doi.org/10.1590/fst.25317
36. De Souza O K, Moura H F C, Brito, Edy Sousa, de e Miranda A R M (2014) Compostos antioxidantes e atividade antioxidante total em frutos de acerola da cv. Flor Branca, Florida Sweet e BRS 366. Rev. Bras. Frutic 36: 294-304. https://dx.doi.org/10.1590/0100-2945-410/13
37. Jost W, Vasil G, Haas C, Thomas B and Atanas P. Ploidy levels in Beta vulgaris (red beet) plant organs and in vitro systems (2010) Engineering in Life Sciences. 10: 139-147.
https://doi.org/10.1002/elsc.200900021
38. LT Evans. Environmental Control of Plant Growth. Elsevier (1963) https://doi.org/10.1016/B978-0-12-244350-3.X5001-0
39. Solar A, Colarič M, Usenik V and Stampar F. Seasonal variations of selected flavonoids, phenolic acids, and quinones in annual shoots of common walnut (Juglans regia L.) (2006) Plant Science 170: 453–461. https://doi.org/10.1016/j.plantsci.2005.09.012
40. Wahba HE, Sarhan AZ, Salama AB, Sharaf-Eldin MA and Gad HM et al. Effect of seasonal variation on the growth and chemical composition of Cynara cardunculus L. plants (2017) J. Mater. Environ. Sci 8: 318-323.
41. Taleb-Contini H S, Schorr K, de Costa B F and de Oliveira RCD. Detection of flavonoids in glandular trichomes of Chromolaena species (Eupatorieae, Asteraceae) by reversed-phase high-performance liquid chromatography (2019) Rev. Bras. Cienc. Farm 43: 315-321. http://dx.doi.org/10.1590/S1516-9332200700020001
42. De Moura OKA, De Oliveira GV, Batalini C, Juliana Aline Rosalem and Ribeiro SL. Antimicrobial activity and quantification of total flavonoids and phenols in different extracts of Propolis (2012) Semina: Biological and Health Sciences 33: 211–222 http://dx.doi.org/10.5433/1679-0367.2012v33n2p211
43. Simões-Ambrosio LMC, Gregório LE, Sousa JPB, Figueiredo-Rinhel and Azzolini AECS et al. The role of seasonality on the inhibitory effect of Brazilian gn propolis on reethe oxidative metabolism of neutrophils (2010) Fitoterapia 81: 1102-1108. https://doi.org/10.1016/j.fitote.2010.07.008
44. Farrant JM, Lehner A, Cooper K and Wiswedel S. Desiccation tolerance in the vegetative tissues of the fern Mohria caffrorum is seasonally regulated (2009) The Plant Journal 57: 65-79. https://doi.org/10.1111/j.1365-313X.2008.03673.x
45. Marslin G, Siram K , Maqbool Q, Selvakesavan KR and Kruszka D et al. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles (2018) 11: 940. https://doi.org/10.3390/ma11060940
*Corresponding author: Bianca Pizzorno Backx, University of Federal University of Rio de Janeiro, NUMPEX-Bio, Campus Duque de Caxias, Brazil, E-mail: biapizzorno@caxias.ufrj.br
Citation: Santos DSM, Santos DLAO, Filho AS, Santana SDCJ, de Souza MF et al., Can green synthesis of nanoparticles be efficient all year long? (2019) Nanomaterial Chem Technol 1: 32-36
Keywords
Nano Materials, Metal Nanoparticles, Green Synthesis, Protocols, Microorganisms


 PDF
PDF