Research Article :
Taís Hartz,
Daiane Fischer, Karina de Fraga and Carla Scheeren Palladium nanoparticles (ca. 4.8 nm) were
synthesized in presence of 1-n-butyl-3-methylimidazolium tetraflouroborate
(BMI.BF4) and 1-n-butyl-3-methylimidazolium hexafluorophosphate
(BMI.PF6) and PMI.Si.(OMe)3.Cl functionalized ionic
liquids using the sol-gel method. The characteristics of the sol-gel method,
ionic liquid on the palladium content was studied, as well as the silica
morphology and texture of the support and the hydrogenation activity. The
palladium content in the resulting xerogels (ca. 0.22 wt% Pd/SiO2)
was shown to be independent of the sol-gel process. The xerogels synthesized in
acidic conditions formed materials with larger pore diameters, which in turn
might be responsible for the higher catalytic activity in hydrogenation of the
alkenes and arenes obtained with the heterogeneous catalyst (Pd/ILs/SiO2). Palladium
catalysts supported are used in various processes including amination, Heck and
Suzuki coupling, hydrogenation,
hydrogen production and dehydrogenation
reactions [1-15]. The Pd/SiO2 is a classical model of Pd catalysts
with SiO2 as the “inert” oxide support [16]. SiO2 resists to reduction and has
low surface acidity, making it relatively inert compared to other oxide
supports, such as TiO2 and Al2O3 [17]. These characteristics make Pd/SiO2 an
ideal starting point for study of the catalytic role of Pd [18,19]. It is well
known that several steps in the catalyst preparation process strongly influence
particle size, including the support composition, metal salt, precursor
deposition method, metal loading, pH, drying conditions, calcinations temperature,
and reduction temperature, among others [20,21]. The combination of an ionic
liquid with a solid support material is emerging as a new alternative for the
immobilization of transition metal catalyst precursors [22,23]. Imidazolium
Ionic Liquids (ILs) possess pre-organized structures mainly through hydrogen
bonds which induce structural directionality [24]. These IL structures can
adapt or be adaptable to many species, as they provide hydrophobic or
hydrophilic regions, and a high directional polarizability
[25,26]. This structural organization of ILs can be used as “entropic drivers”
for spontaneous, well-defined and extended ordering of nanoscale structures.
Indeed, the unique combination of adaptability towards Other molecules and
phases associated to the strong hydrogen-bond driven structure makes ionic
liquids potential key tools in the Preparation of a new generation of chemical
nanostructures such as template porous silica prepared in a sol-gel process
[27-30]. The Metal Nanoparticles
(MNPs) with small diameter and narrow size distribution can be prepared by
simple H2 reduction of metal compounds or decomposition of organometallic
species dissolved in ILs [31-32]. In several cases the MNPs are not stable and
tend to aggregate [33]. Alternatively, these nanoparticles can be used in
conjunction with other stabilizers or be easily transferred to other organic
and inorganic
supports to generate more stable and active catalysts [34-37] The metal
nanoparticles/ionic liquid/stabilizer combination usually exhibits an excellent
synergistic effect that enhances activity of the catalyst. So could be prepared
more efficient and stable catalytic systems using the generation of metal
nanoparticles associated with silica using ILs as templates for both catalytic
partners i.e. the metal nanoparticles and the silica support [38-42]. We
present herein our results, which show that palladium nanoparticles synthesized
in BMI.BF4, BMI.PF6 and PMI.Si.(OMe)3.Cl ionic liquids can be applied for the
generation of the heterogeneous
catalyst (Pd/ILs/SiO2) via sol-gel processes. The heterogeneous catalyst
formed (Pd/ILs/SiO2) was applied in hydrogenation reactions studies. All
experiments were performed in air, except for the synthesis of the Pd NPs. The
Pd NPs and the halide-free BMI.PF6, BMI.BF4 and PMI.Si.(OMe)3.Cl ionic liquids
were prepared according to literature procedure [33,43]. Solvents, alkenes, and
arenes were dried with the appropriate drying agents and distilled under argon
prior to use. All other chemicals were purchased from commercial sources and
used without further purification. Gas
chromatography analysis was performed with a Hewlett-Packard-5890 gas
chromatograph with an FID detector and a 30 m capillary column with a dimethylpolysiloxane
stationary phase. The nanoparticles formation and hydrogenation reactions were
carried out in a modified Fischer–Porter bottle immersed in a silicone oil bath
and connected to a hydrogen tank. The temperature was maintained at 75° C by a
hot-stirring plate. Synthesis
of Palladium Nanoparticles (Pd NPs) supported in silica and ILs: Silica supporting Pd
NPs/ILs/SiO2 were prepared by the solgel method under acidic conditions.
Typical procedure for acid catalysis: 10 mL of tetraethoxy orthosilicate (9.34
g, 45 mmol) was introduced in a Becker under vigorous stirring at 60° C. The Pd
NPs/ILs (10 mg, 0,05 mmol) dispersed in BMI.PF6, BMI.BF4 and PMI.Si.(OMe)3.Cl
ionic liquids (1 mL, 5.1 mmol) and ethanol (5 mL). This solution was submitted
to stirring and sonication for 2 min and then added to the solution containing
TEOS. Consecutively, an acid solution (HF) was added as acid catalyst. The
temperature was kept at 60° C for 18 h. The resulting material was washed
several times with acetone and dried under vacuum. Typical procedure for base
catalysis: 10 mL of TEOS (9.34 g, 45 mmol) was added to ethanol (5 mL),
containing the ionic liquids (1 mL, 5.1 mmol) and previously isolated Pd NPs
(10 mg, 0.05 mmol). Then ethanol (95 mL) and ammonium
hydroxide (20 mL) were added. The mixture was kept under stirring for 3 h
at room temperature and left to stand for a further 18 h. The resulting xerogel
was filtered and washed with acetone and dried under vacuum for 1 h. X-Ray
Diffraction (XRD) The phase
structures were characterized by of XRD Pd NPs. For XRD analysis, the
nanoparticles were isolated as a fine powder and placed on the specimen holder.
The XRD experiments were performed in a SIEMENS D500 diffractometer equipped
with a curved graphite crystal using radiation Cu K ∞ (λ = 1.5406 Å). The
diffraction data were collected at room temperature in Bragg-Brentano geometry ϴ-2
ϴ. The equipment was operated at 40 kV and 20 mA with a scan range between 20°
and 90°. The diffractograms were obtained with a constant step Δ2ϴ = 0.05. The
indexation of Bragg reflections was obtained by fitting a pseudo-Voigt profile
using the code FULPROFF code.37 Nanoparticles Pd/ILs/SiO2 were analyzed on a
glass substrate. Elemental
analysis (CHN) The organic phases
present in the xerogels
were analyzed using CHN elemental Perkin Elmer elemental CHNS/O analyzer, model
400. Triplicate analysis of the samples, previously heated at 100° C under
vacuum for 1 h, was carried out. Rutherford
Backscattering Spectrometry (RBS) Palladium loadings
in catalysts were determined by RBS using He+ beams of 2.0 MeV incidents on
homogeneous tablets of the compressed (12MPa) catalyst powder. The method is
based on the determination of the number and energy of the detected particles,
which are elastically scattered in the Coulombic field of the atomic nuclei in
the target. In this study, the Pd/Si atomic ratio was determined by the heights
of the signals corresponding to each of the elements in the spectra and
converted to wt% Pd/ILs/SiO2. For an introduction to the method and
applications of this technique, the reader is referred elsewhere. Nitrogen
adsorption-desorption isotherms The
adsorption–desorption isotherms of previous degassed solids (150° C) were
determined at liquid nitrogen boiling point in a volumetric apparatus, using
nitrogen as probe. The specific surface areas of xerogels were determined from
the t-plot analysis and pore size distribution was obtained using the BJH
method. A homemade equipment with a vacuum line system employing a
turbo-molecular Edwards vacuum pump was used. The pressure measurements were
made using a capillary Hg barometer and a Pirani gauge. Scanning
Electron Microscopy (SEM) and Electron Dispersive Spectroscopy (EDS) elemental
analysis The materials were
analyzed by SEM using a JEOL model JSM 5800 with 20 kV and 1000 magnification.
The same instrument with was used for the EDS with a Noran detector (20 kV and
acquisition time of 100 s and 1000 magnification). Transmission
Electron Microscopy (TEM) analysis The morphologies
and the Electron Diffraction (ED) patterns of the obtained particles were
determined on a JEOL JEM-2010 equipped with an EDS system and a JEOL JEM-120
EXII electron microscope, operating at accelerating voltages of 200 and 120 kV,
respectively. The TEM samples were prepared by deposition of the Pd NPs or
Pd/ILs/SiO2 isopropanol dispersions on a carbon-coated copper grid at room
temperature. The histograms of the nanoparticle size distributions were
obtained from the measurement of around 300 diameters and reproduced in
different regions of the Cu grid assuming spherical shapes. Catalytic
Hydrogenations
The catalysts (150
mg) were placed in a Fischer–Porter bottle and the alkene or arene (12.5 mmol)
was added. The reactor was placed in an oil bath at 75° C and hydrogen was
admitted to the system at constant pressure (4 atm) under stirring until the
consumption of hydrogen stopped. The organic products were recovered by
decantation and analyzed by GC. The sol-gel
process involves a chemical approach for the synthesis of stable oxide
materials, this process allows us to obtain solid products by creating an oxide
network via progressive polycondensation reactions in a liquid medium [41]. The
steps involved consist of hydrolysis and condensation. The reactions are
affected by the nature of the catalyst. Therefore, in the present study, two main
routes were evaluated: (i) an acid-catalyzed one using either HF, or (ii) a
basecatalyzed approach, using NH4OH as catalyst. In both routes, the hydrolysis
and condensation of Tetraethoxy
Orthosilicate (TEOS) were performed in the presence of Pd NPs, which were
prepared by hydrogen reduction (4 atm) of Pd2(dba)3 dissolved in the ionic
liquids at 75° C [27]. These nanoparticles obtained presented 4.8 nm of
diameter. Figure 1 shows the XRD pattern of Pd NPs and encapsulated in silica
matrix showing the diffraction planes of silica and Platinum (Pd/ILs/SiO2).
This material was obtained by sol-gel synthesis under acidic conditions using
the liquids amount of Pd (0) < 0.2% compared to silica. These Pd
nanoparticles were first isolated from the IL to be characterized by XRD
(Figure 1A). Through the analysis of X-Ray Diffraction (XRD), is possible
identified crystalline palladium in the isolated powder. The characteristics
diffraction lines (111, 220, 200, 311) of metallic Pd can be observed in the diffraction
pattern (Figure 1A). The Pd NPs obtained presented 4.8±0.4 nm with a narrow
range of diameter distribution. The Figure 1B show the Pd/ILs/SiO2 XRD,
diffraction lines (111 and 220) were detected in the sample. TEM analysis of
the synthesized Pd NPs show that the particles display a spherical shape. The
mean diameter observed was 4.8±0.4 nm Pd NPs estimated from ensembles of 300
particles found in an arbitrary chosen area of the enlarged micrographs. The
evaluation of their characteristic diameter results in a monomodal particle
size distribution (Figure 2A). Figure 2B show the obtained particle size
distributions that can be reasonably well fitted by a Gaussian curve. The
rate of condensation slows down with increasing number of siloxane linkages
around a central silicon atom. This leads to formation weakly branched
polymeric networks. The condensation, in case of basic conditions, is
accelerated relative to hydrolysis. The rate of condensation increases with
increasing number of siloxane bridges, result in highly branched networks are
formed [42,43]. In the present case, based on the carbon and nitrogen contents,
it seems that the resulting weakly branched structure generated in the presence
of acid catalyst (either HF) guarantees the constraint of the ionic liquids.
Rutherford Backscattering Spectrometry (RBS) was used in the determination of
the metal contents. The Table 1 show that the immobilized Pd content is roughly
the same for silica prepared by both routes, corresponding to ca. 65-75% of the
initial Pd content employed in the synthesis.
The metal distribution in the support was determined by SEM-EDX analyses.
Mapping showed a homogeneous Palladium distribution in the silica grains,
independently of the preparative route. The Figure 2 shows SEM micrograph
of Pd/ILs/SiO2 synthesized using acid conditions by sol-gel method. The
micrograph show lighter regions, indicating the presence of platinum metal
nanoparticles on the silica matrix (gray regions). The elemental composition of
the region focused on the micrograph confirms this structure. Samples
Pd/ILs/SiO2 were analyzed by the scanning point and area exposed to the
electron beam. All selected areas showed the presence of palladium in the
silica matrix.
In the micrograph, the metal is identified by the bright regions in contrast to
the array of silicon that has the dark background. Figure 3 illustrates the
micrography of Pd/ILs/SiO2 prepared by both routes, acid and basic. According
to Figure 3, particle morphologies are in accordance to that usually observed
for pure silica synthesized by these routes. In the case of acid-catalyzed
conditions, a less organized, platelike structure was observed, while in the
case of basic conditions, spherical particles were obtained. It is worth noting
that smaller particles were produced in the latter case. The textural
properties were further characterized by nitrogen adsorption. Specific area was
calculated by the BET method, while pore diameter, by the BJH one (Table 2).
According to Table 2, silica prepared in the absence of palladium present
higher specific area (ca. 100 m2 g −1 ). The introduction of nanoparticles
during the synthesis, independently of the synthetic route, led to a reduction
in the specific area. The pore diameter was demonstrated to be smaller for the materials
was used NH4OH as catalyst. The pore volume was shown to be independent of the
presence of Pd in acidic or basic conditions. The supported catalysts were
evaluated in hydrogenation reactions. Table 3 presents data regarding 1-decene,
cyclohexene and benzene hydrogenation reactions. For comparative purposes we
also included the data concerning the catalytic activity of isolated Pd NPs
[27] Table 2:Surface area, pore volume and average pore diameter of SiO2/ILs supporting PdNPsa. A)
Reactions
conditions: sol-gel method, constant hydrogen pressure (4 atm), 75 °C ratio
[alkene/Arene]/[Pd/SiO2] = 1250/1, added Pd/SiO2 (150 mg, 0.010 mol Pd NPs
followed by 12.5 mmol of alkenes or arenes. B) grafting method constant
hydrogen pressure (4 atm), 75 °C ratio [alkene/Arene]/Pd/SiO2] = 625/1, SiO2
added (150 mg, 0.025 mmol Pd followed by 12.5 mmol the arenes used. C) Pd
nanoparticles (5 mg. Relation [Arene]/[Pd]] = 250/1, added Pd (5 mg. D) Pd NPs
[alkene/arene]/[metal(0)]= 250/1) followed by 12.5 mmol of alkenes or arenes. Table 3 shows the results
obtained in the hydrogenation reactions using the system Pd/SiO2. Is possible
to observe that all the supported systems were more active than those
constituted of isolated Pd NPs were. Among the silica-based systems, those
prepared under acidic conditions are the most active, exhibiting higher TOF in comparison to those of isolated Pd NPs. The denser and bulkier
structure generated under basic conditions might have afforded less active
systems as shown by some clues. First, the ionic liquids content, which seems
to be important in order to guarantee stability for the nanoparticles, was lower
for these systems. Besides, according to porosimetric measurements, the pore
diameter was much smaller for the SiO2/ILs/PdNPs/NH4OH system. Palladium
encapsulated particles, in spite of a slightly higher content in comparison to
that afforded with an acid catalyst (Table 3), might be not accessible in the
supported systems prepared under basic conditions. The hydrogenation of simple arenes and alkenes by SiO2/ILs/Pd NPs/HF depends on steric hindrance
at the C=C double bond and follows the same trend as observed with classical
palladium complexes in homogeneous conditions, that is, the reactivity follows
the order: terminal-internal. Conclusions Palladium
nanoparticles dispersed in ionic liquids and functionalized ionic liquids
(SiO2/ILs/Pd NPs) can be easily immobilized within a silica network when
prepared by the sol-gel method (acid or base catalysis). The palladium content
in the resulting xerogels was shown to be independent of the preparative route,
but acidic conditions afforded higher encapsulated ionic liquid content and
xerogels with larger pore diameter, which in turn might have guaranteed higher
catalyst activity in the hydrogenation of arenes and alkenes.
The use of ionic liquids for the preparation of both nanoparticles and silica
affords encapsulated SiO2/ILs/Pd NPs materials with different morphology,
texture, and catalytic activity. This combination exhibits an excellent
synergistic effect that enhances the stability
and activity of the Pd NPs in hydrogenation catalysts. All the supported
systems were more active than that constituted of isolated Pd NPs for the
hydrogenation of arenes and alkenes. In particular, the silica-based systems
prepared under acidic conditions were shown to be the most active, exhibiting
higher TOF. The denser and bulkier silica structure generated under basic
conditions (less active catalytic system) incorporated less ionic liquids.
A high level of ionic liquids incorporation seems to be important in order to guarantee
stability for the palladium nanoparticles. 1.
Mehnert CP. Supported ionic liquid catalysis (2004) Chem Eur J 11: 50-56. https://doi.org/10.1002/chem.200400683 2.
Faria VW, Scheeren
CW, Rosa GR, Kurz MHS, Gonçalves FF, et al. Palladium
nanoparticles supported in a polymeric membrane: an efficient phosphine-free
“green” catalyst for suzuki-miyaura reactions in water (2014) RSC
Advances: an intern j to further the chem sci 4: 13446-13452. https://doi.org/10.1039/c4ra01104j 3. Scheeren CW, Fischer
DK and Fraga KR. Chitosan
microspheres from shrimp waste supporting pd nanoparticles in ionic liquids: an
efficient and eco-friendly catalyst for hydrogenation reactions (2020) J of
Nanoscience and Nanotechnology 20: 1296-1302. https://doi.org/10.1166/jnn.2020.16964 4.
Riisager A, Fehrmann R, Haumann M and Wasserscheid P. Supported
ionic liquids: versatile reaction and separation media (2006) Top Catal 40: 91. 5.
Sharma AS, Kaur H and Shah D. Selective oxidation of alcohols by
supported gold nanoparticles: recent advances (2016) RSC Adv 6: 28688-28727. https://doi.org/10.1039/c5ra25646a 6.
Rioux RM, Song H, Grass M, Habas S, Niesz K, et al. Monodisperse
platinum nanoparticles of well-defined shape: synthesis, characterization,
catalytic properties and future prospects (2006) Top catal 39: 167-174. 7.
Verga LG, Russell A and Skylaris CK. Ethanol, O, and co adsorption
on pt nanoparticles: effects of nanoparticle size and graphene support (2018) Phys Chem Chem Phys 20: 25918-25930. https://doi.org/10.1039/c8cp04798g 8.
Riisager
A, Fehrmann R, Haumann M and Wasserscheid P. Supported ionic liquid phase
(silp) catalysis: an innovative concept for homogeneous catalysis in continuous
fixed-bed reactors (2006) Eur J Inorg Chem 695. https://doi.org/10.1002/ejic.200500872 9.
Schmies
H, Bergmann A, Hornberger E, Drnec J, Wang G, et al. Anisotropy of pt
nanoparticles on carbon- and oxide-support and their structural response to
electrochemical oxidation probed by in situ techniques (2020) Phys Chem Chem Phys 22:
22260-22270. https://doi.org/10.1039/d0cp03233f 10.
Maity N, Sahoo A, Boddhula R, Chatterjee S, Panda BB, et al. Fly
ash supported pd–ag bimetallic nanoparticles exhibiting a synergistic catalytic
effect for the reduction of nitrophenol (2020) Dalton Trans 49: 11019-11026. 11. Mehnert
CP, Cook RA, Dispenziere NC and Afeworki M. Supported Ionic Liquid Catalysis -A
New Concept for Homogeneous Hydroformylation Catalysis (2002) J Am Chem Soc
124: 12932-12933. https://doi.org/10.1021/ja0279242 12.
Brett GL, Miedziak PJ and Dimitratos N. (2012) Catal Sci Technol
2: 97-104. 13.
Webb PB, Kunene TE and Cole-Hamilton DJ. Continuous flow
homogeneous hydroformylation of alkenes using supercritical fluids (2005) Green
Chem 7: 373. 14. Miyazaki
A, Matsuda K, Papa F, Scurtu M, Negrila C, et
al. Impact of particle size and metal–support interaction on denitration
behavior of well-defined Pt–Cu nanoparticles (2015) Catal Sci Technol 5:
492-503. https://doi.org/10.1039/C4CY00929K 15.
deCastro C, Sauvage E, Valkenberg Mh and Holderich WF. Immobilised Ionic Liquids
as Lewis Acid Catalysts for the Alkylation of Aromatic Compounds with Dodecene
(2000) J
Catal 196: 86-94. https://doi.org/10.1006/jcat.2000.3004 16.
Mehnert CP, Mozeleski EJ and Cook RA. Supported ionic liquid
catalysis investigated for hydrogenation reactions (2002) Chem Commun
3010-3011. DOI https://doi.org/10.1039/B210214E 17.
Hagiwara H, Sugawara Y, Isobe K, Hoshi T and Suzuki T.
Immobilization of Pd(OAc)(2) in ionic liquid on silica: application to
sustainable Mizoroki-Heck reaction (2004) Org Lett 6: 2325-2328. https://doi.org/10.1021/ol049343i 18.
Breitenlechner
S, Fleck M, Muller TE and Suppan A. Solid catalysts on the basis of supported
ionic liquids and their use in hydroamination reactions (2004) J Mol Catal A
Chem 214: 175-179. https://doi.org/10.1016/j.molcata.2003.12.032 19.
Dupont J
and Suarez PAZ. Physico-chemical processes in imidazolium ionic liquids (2006)
Phys Chem Chem Phys 8: 2441. https://doi.org/10.1039/b602046a 20.
Consorti
CS, Suarez PAZ, de Souza RF, Burrow RA, Farrar DH, et al. CCDC 268404:
Experimental Crystal Structure Determination (2005) J Phys Chem B 109: 4341. 21.
Dupont J.
On the solid, liquid and solution structural organization of imidazolium ionic
liquids (2004) J Braz Chem Soc 15: 341. https://doi.org/10.1590/S0103-50532004000300002 22.
Atonietti
M, Kuang DB, Smarsly B and Yong Z. Ionic 23.
Zhou Y
and Antonietti M. Synthesis of very small tio2 nanocrystals in a
room-temperature ionic liquid and their self-assembly toward mesoporous
spherical aggregates (2003) J Am Chem Soc 125: 14960-14961. https://doi.org/10.1021/ja0380998 24.
Zhou Y,
Schattka JH and Antonietti M. Room-temperature ionic liquids as template to
monolithic mesoporous silica with wormlike pores via a sol-gel nanocasting
technique (2004) Nano Lett 4: 477-481. https://doi.org/10.1021/nl025861f 25.
Jin R ,
Zeng C, Zhou M and Chen Y. Atomically precise colloidal metal nanoclusters and
nanoparticles: fundamentals and opportunities (2016) Chem Rev 116: 10346-413. 26. Dai S, Ju YH, Gao HJ, Lin JS, Pennycook SJ, et al.
Preparation of silica aerogel using ionic liquids as solvents (2000) Chem
Commun 243-244. https://doi.org/10.1039/a907147d 27.
Scheeren
CW, Machado G, Teixeira SR, Morais J, Domingos Jb, et al. Synthesis and
characterization of Pt0 nanoparticles in imidazolium ionic liquids (2006) J
Phys Chem B 110: 13011-13020. https://doi.org/10.1021/jp0623037 28.
Silveira
ET, Umpierre AP, Rossi LM, Machado G, Morais J, et al. The partial
hydrogenation of benzene to cyclohexene by nanoscale ruthenium catalysts in
imidazolium ionic liquids (2004) Chem Eur J 10: 3734-3740. https://doi.org/10.1002/chem.200305765 29.
Dupont J,
Fonseca Gs, Umpierre AP, Fichtner PFP and Teixeira SR. Transition-metal
nanoparticles in imidazolium ionic liquids: recyclable catalysts for biphasic
hydrogenation reactions (2002) J Am Chem Soc 124: 4228-4229. https://doi.org/10.1021/ja025818u 30.
Dupont
J and Migowski P. Catalytic applications of metal nanoparticles in imidazolium
ionic liquids (2007) Chem Eur J 13: 32-39. https://doi.org/10.1002/chem.200601438 31.
Mu XD,
Evans DG and Kou YA. A general method for preparation of PVP-stabilized noble
metal nanoparticles in room temperature ionic liquids (2004) Catal Lett 97:
151-154. 32. Mu XD, Meng JQ, Li ZC and Kou Y. Rhodium nanoparticles
stabilized by ionic copolymers in ionic liquids: long lifetime nanocluster
catalysts for benzene hydrogenation (2005) J Am Chem Soc 127: 9694-9695. https://doi.org/10.1021/ja051803v 33.
Miao SD,
Liu ZM, Han BX, Huang J, Sun ZY, et al. Ru nanoparticles immobilized on
montmorillonite by ionic liquids: a highly efficient heterogeneous catalyst for
the hydrogenation of benzene (2006) Chem Int Ed 45: 266-269. 34. Huang J, Jiang T, Han B and Wu W. A novel method to
immobilize ru nanoparticles on sba-15 firmly by ionic liquid and hydrogenation
of arene (2005) Catal letters 103: 59-62. 35. Mevellec V, Nowicki A, Roucoux A, Dujardin C, Granger
P, et al. A simple and reproducible method for the synthesis of
silica-supported rhodium nanoparticles and their investigation in the
hydrogenation of aromatic compounds (2006) New J Chem 30: 1214-1219. https://doi.org/10.1039/b605893k 36.
Li S, Liu
M, Zhang A and Guo X. Spherical mesoporous silica templated with ionic liquid
and cetyltrimethylammonium bromide and its conversion to hollow spheres (2010)
materials letters 64: 599-601. https://doi.org/10.1016/j.matlet.2009.12.013 37.
Moseley K
and Maitlis PMJ. Bis- and
tris-(dibenzylideneacetone)platinum and the stabilization of zerovalent
complexes by an unsaturated ketone (1971) Chem Soc Chem Commun 982-983. https://doi.org/10.1039/c29710000982 38.
Cassol
CC, Ebeling G, Ferrera B and Dupont J. A simple and practical method for the
preparation and purity determination of halide-free imidazolium ionic liquids
(2006) Adv Synth Catal 348: 243. https://doi.org/10.1002/adsc.200505295 39.
Carvajal
JR. Introduction to the program fullprof. laboratoire leon brillon (cea-cnrs),
saclay france (2004) 40.
Schubert
U and Husing N. Inorganic Materials: A Chemical Approach 1st edn
Wiley Weinheim 2000. 41.
Bernardi
F, Alves
MCM, Traverse A, Silve DO, Scheeren CW, et al. (2009) J Phys Chem 113: 3909. 42. Vansant
EF, Van Der Voort P and Vrancken KC. Characterization and Chemical Modification of
the Silica Surface.
Carla Scheerena Laboratory of Catalysis, School of
Chemistry and Food, Federal University of Rio Grande - FURG, Rua Barão do Caí,
125, CEP 95500-000, Santo Antônio da Patrulha, RS, Brazil. Email: carlascheeren@gmail.com
Hartza T, Fischera D, de Fragaa K and Scheerena C.
Ionic liquids/SiO2 supporting Pd nanoparticles: efficient catalysts in
hydrogenation reaction (2021) Nanomaterial Chem Technol 2: 8-12. Nanoparticles, Material, ChromatographyIonic Liquids/SiO2 Supporting Pd Nanoparticles: Efficient Catalysts in Hydrogenation Reaction
Abstract
Full-Text
Introduction
Experimental
style="text-align:justify">GeneralResults
and Discussion
style="margin-bottom: 0.0001pt;">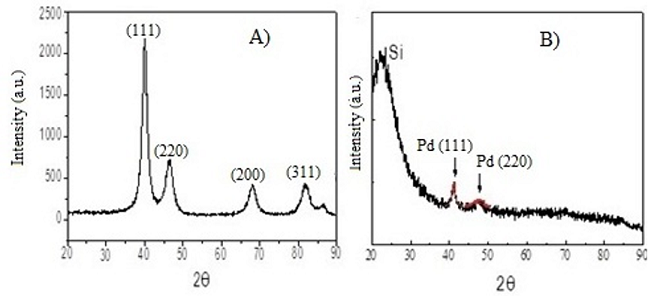
Figure1: XRD analysis of: A) Pd NPs (4.8 nm) and B) Pd NPs/IL/SiO2.
Table 1:Elemental analysis of samples Pd/ILs/SiO2
Transmission Electron Microscopy
(TEM) was also employed for the characterization of the supported catalyst.
Figure 4 shows the micrograph of the Pd/ILs/SiO2, the mean size of which was
shown to be ca. 4.8 nm. It is very likely that the presence of ionic liquids
affords stability, avoiding sintering of the metallic particles.
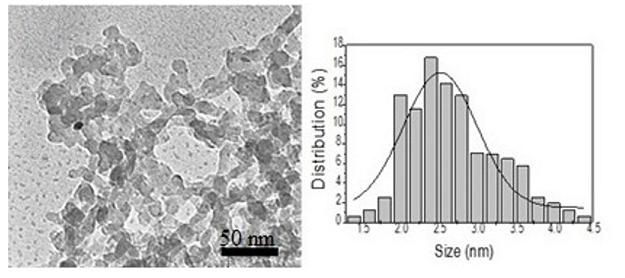
Figure 4: Micrographs obtained by TEM of Pd/ILs/SiO2/HF and histogram of diameter distribution.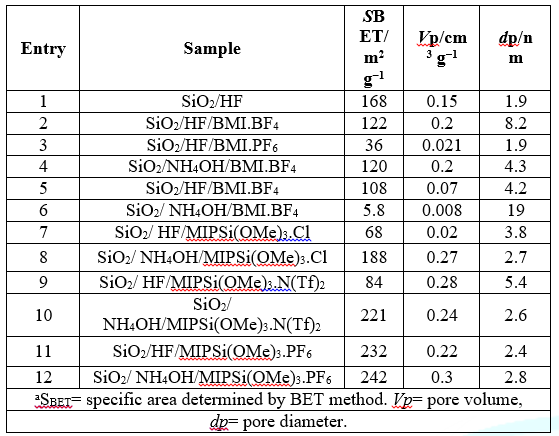
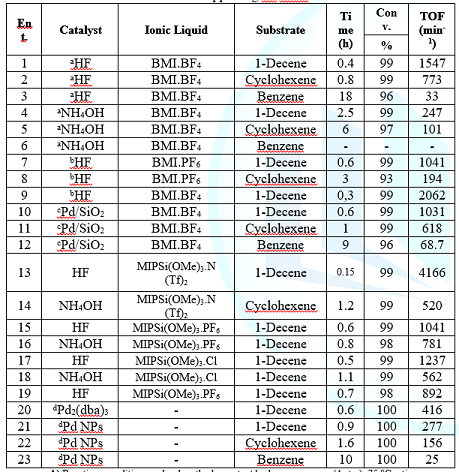
Table3: Hydrogenation of alkenes by encapsulated Pd/ILs/SiO2a and Pd NPsb. Acknowledgements
Thanks are due to the following Brazilian Agencies:
CNPq, CAPES, FAPERGS for fellowships and partial financial support.References
Corresponding author
Citation
Keywords