Research Article :
Gao Yan,
Li Ling, Zhao Fang, ChenXiao Yuan and ChenChuan
Ying Background: Masked Hypertension (MHT) is associated with an
increased risk for Cardiovascular Disease (CVD). The etiopathogenesis of MHT is
thought to be affected by oxidative stress and vascular inflammation. This
study aimed to analyze the relationships between Lipoprotein-Associated
Phospholipase A2 (Lp-PLA2), a unique vascular inflammation marker, with blood
pressure variation and traditional risk factors in patients with MHT, and to
determine the clinical significance. Methods: One hundred eighty-three patients without any prior
therapeutic medications were included and divided into the following three
groups: MHT (n=82); True Hypertension (THT) [n=52]); and normotensive (n=59).
An Ambulatory Blood Pressure Monitor (ABPM) was used. Clinical biochemical
parameters and the Lp-PLA2 mass in each group were measured, and the related
clinical characteristics and risk factors for CVD were statistically analyzed. Results: The level of Lp-PLA2 in MHT group was
significantly higher than the normotensive (191.8 ± 62.58 vs.108.3 ± 44.74
ng/ml, p<0.01) and true hypertension groups (191.8 ± 62.58 vs. 169.3 ± 54.55
ng/ml, p<0.05). Furthermore, the incidence of MHT was correlated with the
increase in Lp-PLA2, around 65% of MHT patients with a Lp-PLA2 level ≥ 225 μ mol/L.
The Lp-PLA2 level had a positive correlation with ABPM measurements,
office-measured systolic blood pressure, and serum Uric Acid (UA) and Low-Density
Lipoprotein Cholesterol (LDL-C) levels, but a negative correlation with the
High-Density Lipoprotein Cholesterol (HDL-C) level. Conclusion:
An increased LP-PLA2 level was closely associated with the metabolic stress and
incidence of MHT, thus exhibit an important role in the pathophysiology and
diagnostic assessment of MHT. Masked Hypertension (MHT) is a
special phenotype of abnormal blood pressure variation associated with
increased Cardiovascular Disease (CVD) risk, and accounts for 30% of
pre-hypertensive patients. In clinical practice, early screening and risk
stratification of CVD for patients with MHT are challenging due to variability
of dynamic blood pressure and unmarked early target organ damage in
hypertension. So, it is necessary to find novel possible biomarkers to comb
with ABPM for screening and diagnostic assessment of the masked hypertension.
Lp-PLA2 is a unique biomarker for vascular inflammation and CVD risk function
as a pro-inflammatory enzyme. Recent studies demonstrated that Lp-PLA2 play a
key role in the proatherogenic effects and development of Atherosclerosis (AS),
which have clinical application value to predict potential cardiovascular
diseases. However, there is limited clinic evidence on the effect of Lp-PLA2 on
Masked hypertension. The relationship between MHT-related inflammation and A2
(Lp-PLA2) has not been reported. The current study aimed to analyze the
association between the plasma Lp-PLA2 mass with blood pressure variation and
traditional risk factors in MHT patients and to determine the clinical
significance of the association [1-6]. Eighty-two patients with MHT (67
males and 15 females, 47.15 ± 14.5 years of age), 52 patients with True
Hypertension [THT] (40 males and 12 females, 43.76 ± 13.8 years of age), and 59
normotensive patients (48 males and 11 females, 47.38 ± 13.8 years of age) were
selected for the present cross-sectional study. None of the patients were
treated pharmacologically. The THT and normotensive patients were age-matched
with the MHT patients. The patients underwent medical evaluation from April
2018 to December 2019 in the Department of Cardiology of Shenzhen Shekou Peoples
Hospital, and defined as MHT, THT, and normotensive groups. All of the patients
signed informed consent. This study was approved by the Shenzhen Shekou Peoples
Hospital. The methods were carried out in accordance with the Declaration of
Helsinki guidelines, including any relevant details [7]. All participants underwent 24-h
ABPM with an automatic blood pressure monitor (Welch Allyn ABPM 6100 device;
Welch Allyn Poland and Baltic States, Poznan, Poland), in accordance with the
International Database of Ambulatory Blood Pressure and Cardiovascular Disease
(IDACO). Daytime was defined as 10 am to 8 pm and nighttime was defined as 12
am (midnight) to 6 am. The device was programmed to obtain Blood Pressure (BP)
readings at 20-min intervals. The recording was then calculated to obtain a 24h
average Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP). When
the readings exceeded at least 70% of the total readings programmed for the
testing period, the recording was considered valid and satisfactory [8]. Blood samples were collected
following a 12-h overnight fast and were used for Lp-PLA2 analysis. Blood
samples (3 milliliter) were obtained from peripheral veins and collected into
tubes with EDTA anticoagulants. The tubes were centrifuged immediately at 3000
r/min for 3 min. The separated upper plasma was stored at -80o C.
Lp-PLA2 analysis was performed every 7 days. The plasma Lp-PLA2 mass was
determined using an ELISA kit (Lp-PLA2 Test Kit; Kangerke Technologies, Inc.,
Tianjin, China) according to the manufacturers instructions. Measurement of
biochemical parameters: Blood samples were collected following a 12-h overnight
fast and were assayed for blood. Samples (5 milliliter) were drawn from
peripheral veins and collected into tubes. The tubes
were centrifuged immediately at 3000 r/min for 10 min. The separated serum
samples were used for biochemical indices analysis using commercially available
kits and an Architect C16000 (Abbott, Lake Forest, Ill, USA), and included a
Fasting Blood Glucose (FBG), lipids, Blood Urea Nitrogen (BUN), Creatinine
(Cr), and Uric Acid (UA). Low-Density Lipoprotein Cholesterol (LDL-C) was
calculated using the Friedewald formula when the Triglycerides (TG) level was ≤
5.0 m mol/l. No patient had a TG level ≥ 5.0 m mol/l [9]. Body Mass Index (BMI) was
obtained by dividing the body weight by the square of the height in meters. The
estimated Glomerular Filtration Rate (eGFR) was calculated using the
Modification of Diet in Renal Disease (MDRD) study equation, as follows: eGFR
(mL/min/1.73m2)=30849 × [Scr (u mol)]-1.154 × (age)-0.203
× 0.742 (if female). According to the 2018 ESC/ESH
guidelines for the management of arterial hypertension, normotension is defined
clinically by an Office Blood Pressure (OBP) <140/90 mmHg and based on the
following ABPM average daytime SBP<135 mmHg and/or DBP<85 mmHg average
nighttime SBP<120 mmHg and/or DBP<70 mmHg and average 24h SBP<130 mmHg
and/or DB <80 mmHg. The diagnostic threshold for THT is defined clinically
by an OBP>140/90 mmHg and based on the following ABPM: average daytime
SBP>135 mmHg and/or DBP>85 mmHg: average nighttime SBP>120 mmHg and/or
DBP>70 mmHg and average 24-h SBP>130 mmHg and/or DBP>80 mmHg.
According to the 2013 European Society guidelines for the management of ABPM,
MHT is clinically defined as an OBP <140/90 mmHg, and masked daytime, masked
nighttime, or masked 24-h hypertension from ABPM are categorized as MHT. Based
on the average of all measurements between 10 am and 8 pm, daytime hypertension
was defined as a SBP ≥ 135 mmHg and/or DBP ≥ 85 mmHg. Based on the mean of all
measurements between 12 am and 6 pm, nighttime hypertension was defined as a
SBP ≥ 120 mmHg and/or DBP ≥ 70 mmHg. Using the average of all available
measurements from ABPM, 24h hypertension was defined as a SBP ≥ 130 mmHg and/or
DBP ≥ 80 mmHg [10,11]. Secondary hypertension; acute
cardio-cerebrovascular disease; heart failure; neoplasm; autoimmune and
rheumatic diseases; pregnancy; acute and chronic infections; severe liver and
kidney dysfunction; thyroid dysfunction; and recent surgical trauma.
Statistical analyses were performed using the SPSS 19.0 (SPSS, Inc., Chicago,
IL, USA). All variables were tested for normal distribution of the data. Data
are presented as the mean ± Standard Deviation (SD) or the count number and
proportion. Differences between the studied groups were examined using the
Students unpaired t-test for parametric data. The categorical data were
examined with a chi-square test. Influencing factors of Lp-PLA2 were found by
Spearman linear regression analysis. All comparisons were 2-sided at the 5%
significance level. A P value <0.05 was considered to be statistically
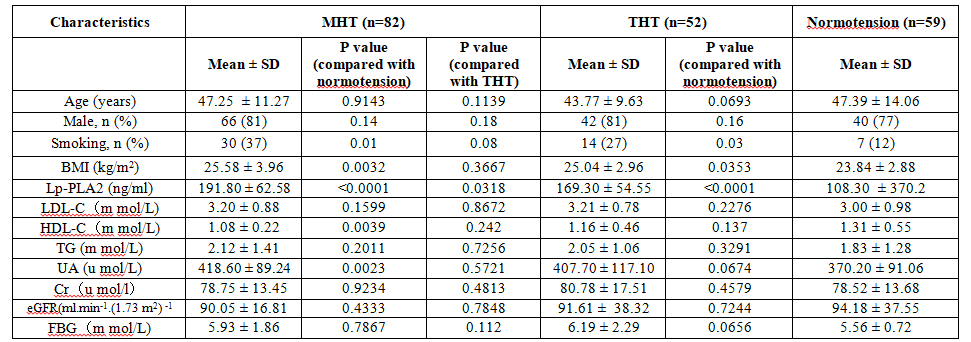
significant. Comparison of clinical
characteristics, demographics, and biochemical indices are presented in Table 1. There were no statistically
significant differences in age, gender, FBG, TG, LDL-C, Total Cholesterol (TC),
Cr, and eGFR among the three groups (P>0.05).The proportion of patients with
smoking history, BMI, Lp-PLA2 level, and serum UA level in the MHT group were
significantly higher than the normotensive group (p<0.05). The level of
HDL-C was significantly lower than the normotensive group (p<0.01). Comparison
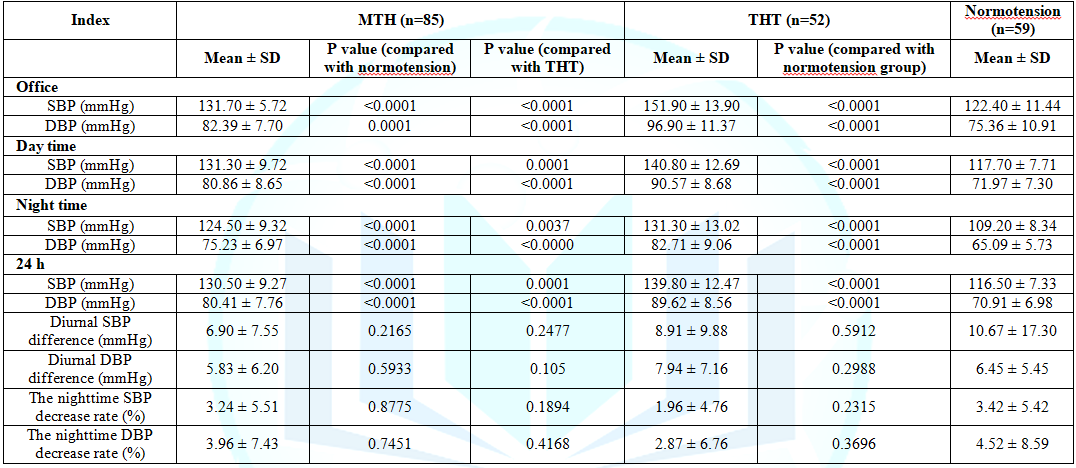
of OBP and ABPM are presented in Table 2.
The mean OBP in the MHT group was significantly lower than the THT group
(p<0.01), and significantly higher than the normotensive group (p<0.01).
The average SBP, average DBP during the daytime, nighttime, and 24-h average in
the MHT group were all significantly higher the normotensive group (p<0.01).
The average diurnal BP difference and the rate of nighttime BP decrease in the
MHT group was lower than the other groups, but there were no statistically significant
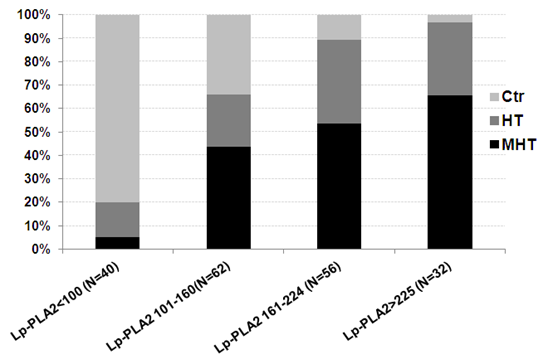
difference among the three groups (p>0.05). Comparison of the incidence of
normotensive, THT, and MHT based on the Lp-PLA2 interquartile range are
presented in Figure 1. The incidence
of MHT was associated with an increase in the plasma Lp-PLA2 mass. Among the
quartiles, the Lp-PLA2 level had the greatest impact on the occurrence of MTH.
Moreover, the occurrence of MHT was up to 65% in patients with an Lp-PLA2 ≥ 225
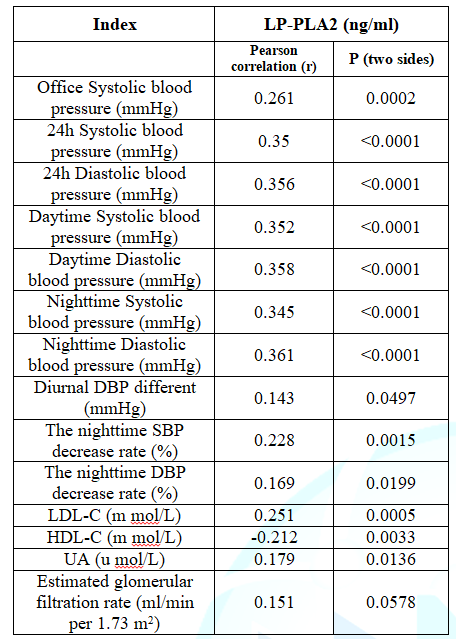
μ mol/L (Figure 1). Spearman linear regression analysis of Lp-PLA2 with BP
measurements and chemical indices were shown in Table 3. The Lp-PLA2 level had a positive
correlation with office SBP, ABPM, and serum UA and LDL-C levels, but a
negative correlation with the HDL-C level. Previous studies had indicated
that MHT was affected by oxidative stress and vascular inflammation. However,
the etiopathogenesis of which remains completely uncertain. Findings from the
current study suggest that that the proportion of patients with smoking,
obesity, and high UA level in the Masked Hypertension (MHT) group was
significantly higher than the normotensive group (p<0.05), rather than Total
Cholesterol (TC), LDL-C and fasting glucose. This is consistent with The
Jackson Heart Study, which suggested that better diet, not smoking and lower
clinic BP were each associated with a lower prevalence of masked daytime
hypertension. More and more clinic evidences demonstrated that high levels of
SUA were an independent risk factor associated with risk of hypertension beyond
traditional risk factors, although the intrinsic mechanism needs further
elucidation. Studies had reported that MHT was independently associated with
increased serum Glycosylated Hemoglobin (HbA1c) and CRP levels, and Type 2
diabetic patients with MHT had higher risk of Target Organ Damage (TOD). However,
in our present study, glycol metabolism assessment was only performed on the
fasting blood glucose. Future trials to measure HbA1c and 2 Hours Postprandial
Blood are warranted to investigate the association between glycol metabolism
and the prevalence of MHT [12-18]. Additionally, the data from
current study also showed that TC and LDL-C levels had no significantly
difference among three groups. Recent studies by Tsimikas et al had demonstrated
that Lp(a)-linked Oxidized Phospholipids (OxPLs) play a key role in the
proatherogenic effects of Lp(a). In a study by Bergmark et al unlike LDL, Lp(a)
had the physiological function of preferentially binding to OxPL in
circulation. Moreover, Lp(a) could be preferentially aggregated to the vascular
lesion site, causing the formation of OxPL and resulting in marked increase of
Lp-PLA2 enzyme activity so as to significant enhancing the atherogenesis.
Lp-PLA2 is another important factor in LP (a) function, which had been proved
to be a special biomarker related to endothelial dysfunction and vascular
inflammation via hydrolysis of OxPL leading to the release of inflammation
mediators [lyso-phos phatidylocholine (lyso-PC) and Oxidized Fatty Acids
(ox-FA) [19,20]. Previous clinical and
epidemiologic studies had demonstrated that Lp-PLA2 activity is an independent
predictor of Coronary Heart Disease (CAD) and stroke beyond traditional risk
factors in the general population. However, there is limited knowledge on the
effect of Lp-PLA2 on early inflammatory cardiovascular damage. Our current
study investigated the relationships between the plasma Lp-PLA2 mass and BP
variation in pre-hypertensive patients without pharmacological treatment. The
results showed that plasma Lp-PLA2 mass in both MHT and THT group were
significantly higher than nomotensive individuals matched for age and sex. It
is worth noting that the incidence of MHT reached 65% among patients with an
Lp-PLA2 ≥ 225 μ mol/L. In agreement with this result, recent studies had
reported that MHT was associated with vascular inflammation-induced endothelial
dysfunction and early arterial damage also provided a clinic evidence of
prehypertension-associated elevations in plasma Lp-PLA2 activity, OxPL, and
lysoPCs [5,6,21-23]. Previous animal experiments
reported by Wang et al demonstrated that Lp-PLA2 participates in OxPL-induced
progression of atherosclerosis in many ways, not only by up-regulating genes
expression of lp-PLA2 and proinflammatory molecules through p38 MAPK pathway in
monocytes, but also by trigging the migration of Smooth Muscle Cell (SMC) and
endothelial cell death by production of lyso-PC, thereby activating the
systemic and localized vascular inflammatory cascade response and contributing
to the development of atherosclerotic lesions. In addition, the hemodynamic
changes induced by blood pressure fluctuations can also cause the vascular
endothelial injury along with inflammation response [24-29]. Interestingly, a novel finding
from current study was that Lp-PLA2 mass in MHT was significantly higher than
the true hypertension group, although there was no difference in the incidence
of carotid plaque between the two groups. Previous clinic studies had reported
MHT had a high degree of recurrence, and pro-inflammation triggered by Lp-PLA2
catalyzed Ox-PLs hydrolysis could prompt arterial stiffness and vascular compliance
in pre-hypertensive patients. Studies by Watanabe et al and Mazzali et al
reported that oxidative stress over activity induced by increased sUA levels
had a detrimental effect on the vascular endothelium and contribute to pressure
fluctuations via stimulating the renin-angiotensin system and promoting acute
retention of water and sodium. Our present study further confirmed that the
plasma Lp-PLA2 mass has a positive correlation with BP variability based on
measurements of ABPM in patients with MHT. Thus it is conceivable that a higher
degree of oxidative stress-dependent inflammatory vascular responses may be
considered to play an important role in pathogenesis of MTH [30-33]. Indeed, this persistent
inflammatory vascular responses and pressure fluctuation had been proved to
induce the development of arteriolosclerosis, renal interstitial fibrosis and
permanent sodium-sensitive hypertension, which were involved in the process of
true hypertension. A study by Sánchez-Lozada LG et al. [34] reported that a constant
mildly hyperuricemia rats could develop renin-dependent hypertension and
interstitial renal disease. So these studies indicated that oxidative stress
and inflammatory response may exert different degree effect on the while
pathophysiology processes of primary hypertension. In clinic practice, focusing
on the baseline and on-treatment level of inflammatory markers in patient with
MHT will better prevent and target the prevalence and development of
hypertension and arteriolosclerosis. Spearman linear regression analysis in
current study revealed that Lp-PLA2 had a positive correlation with traditional
CVD risk factors (UA and LDL-C), but a negative correlation with HDL-C. Chae et
al. [35] also reported a positive association between plasma ox-LDL and Lp-PLA2
activity in metabolic syndrome. Uric acid acts as a useful biochemical marker
of oxidative stress and endothelial function had been shown to be a
well-established driver of local and systemic inflammatory vascular responses
due to production of ox-LDL and pro-inflammatory factors, thereby increasing
Lp-PLA2 activity and concentration, although the intrinsic mechanism needs
further elucidation [36,37]. Similar to the present study,
Theilmeier et al. [38] and Britesa et al. [39] demonstrated by in vitro and in
vivo models that HDL-C could play an anti-atherogenic action via prevents the
accumulation of lipid hydroperoxides in LDL-C. Thus, the increased Lp-PLA2
level in MHT patients may also be associated with suppression of anti-oxidative
activity due to a decrease in HDL-C. Limited by our cross-sectional
and observational study, the potential utility of LP-PLA2 in patients with MHT
as a biomarker in cardiovascular risk prediction and as the therapeutic target
still warrants a prospective study. Because the expression of Lp-PLA2 is
regulated by the PLA2G7 gene, its activity or mass differ in predicting
different diseases. More experimental evidence is needed to fully interpret the
intrinsic functional role of Lp-PLA2 in different inflammatory cardiovascular
diseases. In conclusion, the present study
demonstrated that an increased plasma LP-PLA2 mass is closely associated with
metabolic stress and the incidence of MHT, and provided new evidence that
LP-PLA2 may be involved in dynamic regulation of oxidative stress-dependent
inflammatory vascular responses, thus exerting pathophysiological effect on the
development of MHT. In clinical practice, focusing on the detection and
follow-up of the plasma LP-PLA2 level could facilitate risk stratification and
targeted therapy against atherosclerosis in MHT patients. 1.
Bromfield GS,
Shimbo D, Booth JN, Diaz KM, Correa A, et al. Cardiovascular risk factors and
masked hypertension (2016) Jackson Heart Study Hypertension 68: 1475-1482. https://dx.doi.org/10.1161%2FHYPERTENSIONAHA.116.08308 2. Wang
YC, Shimbo D, Muntner P, Moran AE, Krakoff LR, et al. Prevalence of masked
hypertension among us adults with nonelevated clinic blood pressure (2017) Am J
Epidemiol 185: 194-202. https://dx.doi.org/10.1093/aje/kww237 3.
Stergiou GS,
Ntineri A and Kollias A. Management of masked hypertension why are we still
sitting on the fence? (2016) Hypertension: 68: 1344-1345. https://doi.org/10.1161/HYPERTENSIONAHA.116.08393 4. Wang
J, Tan G, Han L, Bai Y, He M, et al. Novel biomarkers for cardiovascular risk
prediction (2017) J Geriatric Cardiology 14: 135-150. https://doi.org/10.11909/j.issn.1671-5411.2017.02.008
5. Thompson
A, Gao P, Orfei L, Watson S, Di Angelantonio E, et al. Lipoprotein-associated
phospholipase A2 and risk of coronary disease, stroke, and mortality:
collaborative analysis of 32 prospective studies (2010) Lp-PLA(2) Stud Collabo
375: 1536-1544. https://doi.org/10.1016/S0140-6736(10)60319-4 6. Packard
CJ, OReilly DS, Caslake MJ, McMahon AD, Ford I, et al. Lipoprotein-associated phospholipase
A2 as an independent predictor of coronary heart disease (2000) N Engl J Med
343: 1148-1155. https://doi.org/10.1056/NEJM200010193431603
7. Declaration
of Helsinki (2013) J Am Med Assoc 310: 2191-2194. 8. Thijs
L, Hansen TW, Kikuya M, Björklund-Bodegård K, Li Y, et al. The international
database of ambulatory blood pressure in relation to cardiovascular outcome
(IDACO): protocol and research perspectives (2007) Blood Press Monit 12: 255-262.
https://doi.org/10.1038/hr.2012.97
9. Caslake
MJ, Packard CJ, Suckling KE, Holmes SD, Chamberlain P, et al.
Lipoprotein-associated phospholipase A (2), platelet-activating factor
acetylhydrolase: a potential new risk factor for coronary artery disease (2000)
Atherosclerosis 150: 413-419.
https://doi.org/10.1016/s0021-9150(99)00406-2
10. Williams
B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, et al. 2018 ESC/ESH
Guidelines for the management of arterial hypertension. The Task Force for the
management of arterial hypertension of the European Society of Cardiology and
the European Society of Hypertension (2018) J Hypertens 36: 1953-2041. https://doi.org/10.1097/HJH.0000000000001940
11. OBrien
E, Parati G, Stergiou G, Asmar R, Beilin L, et al. European society of
hypertension working group on blood pressure monitoring (2013) J Hypertens 31: 1731-1768. https://doi.org/10.1097/HJH.0b013e328363e964
12. Taner
A, Unlu A, Kayrak M, Tekinalp M, Ayhan SS, et al. The value of serum asymmetric
dimethyl arginine levels for the determination of masked hypertension in
patients with diabetes Mellitus (2013) Atherosclerosis 228: 432-437. https://doi.org/10.1016/j.atherosclerosis.2013.02.024
13. Takeno
K, Mita T, Nakayama S, Goto H, Komiya K, et al. Masked Hypertension,
Endothelial Dysfunction, and arterial stiffness in type 2 diabetes mellitus: A
Pilot Study (2012) Am J Hypertension 25: 165-170. https://doi.org/10.1038/ajh.2011.158
14. Bromfield
SG, Shimbo D, Booth JN, Correa A and Ogedegbe G. Cardiovascular risk factors
and masked hypertension: the jackson heart study (2016) Hypertension 68:
1475-1482. https://doi.org/10.1161/HYPERTENSIONAHA.116.08308
15. Grayson
PC, Kim SY, LaValley M and Hyon KC. Hyperuricemia and incident hypertension: a
systematic review and meta-analysis (2011) Arthritis Care Res 63: 102-110. https://doi.org/10.1002/acr.20344
16. Ndrepepa
G. Uric acid and cardiovascular disease (2018) Clinica Chimica Acta 484: 150-163. https://doi.org/10.1016/j.cca.2018.05.046
17. Zhao
H, Zeng F, Wang X and Wang L. Prevalence, risk factors, and prognostic
significance of masked hypertension in diabetic patients (2017) Medicine 96:
e8363. https://doi.org/10.1097/MD.0000000000008363
18. Leitão
CB, Canani LH, Kramer CK, Boza JC, Pinotti AF, et al. Masked hypertension,
urinary albumin excretion rate, and echocardiographic parameters in putatively
normotensive type 2 diabetic patients (2007) Diabetes Care 30: 1255-1260. https://doi.org/10.2337/dc06-2131
19. Tsimikas
S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, et al. Oxidized phospholipids,
Lp(a), lipoprotein and
coronary artery disease (2005) N Engl J Med 353: 46-57. https://doi.org/10.1056/NEJMoa043175
20. Bergmark
C1, Dewan A, Orsoni A, Merki E, Miller ER, et al. A novel function of
lipoprotein [a] as a preferential carrier of oxidized phospholipids in human
plasma (2008) Lipid Res 49: 2230-2239.
https://doi.org/10.1194/jlr.M800174-JLR200
21. Kim
M, Jung S, Kim S, Lee S and Lee JH. Prehypertension- associated elevation in
circulating lyso phosphatidly cholines, lp-pla2 activity, and oxidative stress
(2014) Plos One 9: e96735.
https://dx.doi.org/10.1371%2Fjournal.pone.0096735
22. Inan
B, Ates I, Ozkayar N, Kundi H, Topcuoglu C, et al. Are increased oxidative
stress and asymmetric dimethyl arginine levels associated with masked
hypertension? (2016) Clin Exp Hypertens 38: 294-298. https://doi.org/10.3109/10641963.2015.1089883
23. Kenny
IE, Saeed S, Gerdts E, Midtbo H, Halland H, et al. Masked hypertension in
obesity: potential predictors and arterial damage (2017) Blood Press Monit 22:
12-17. https://doi.org/10.1097/MBP.0000000000000220
24. Wang
W, Li J, Yang D, Xu W, Zha RP, et al. OxLDL stimulates lipoprotein-associated phospholipase
A2 expression in THP-1monocytes via PI3K and p38 MAPK pathways (2010) Cardiov
Res 85: 845-852. https://doi.org/10.1093/cvr/cvp367
25. Chen
L, Wang W and Wang Y. Inhibitory effects of litho spermic acid on proliferation
and migration of rat vascular smooth muscle cells (2009) Acta Pharmacola Sin
30: 1245-1252. https://doi.org/10.1038/aps.2009.122
26. Marshall
AC, Peter HJ and Michael HD. Review of the evidence for the clinical utility of
lipoprotein-associatedphospholipazeA2 as a cardiovascular risk marker (2008) Am
J Cardiol 10: 41F-50F.
https://doi.org/10.1016/j.amjcard.2008.04.018
27. Yarla
NS, Bishayee A, Vadlakonda L, Chintala R, Duddukuri GR, eta al. Phospholipase
A2 isoforms as novel targets for prevention and treatment of inflammatory and
oncologic diseases (2016) Current Drug Targets 17: 1940-1962. https://doi.org/10.2174/1389450116666150727122501
28. Zalewski
A and Macphee C. Role of Lipoprotein-Associated Phospholipase A2 in
Atherosclerosis: Biology, Epidemiology, and Possible Therapeutic Target (2005)
Arterioscler Thromb Vasc Biol 25: 923-931.
https://doi.org/10.1161/01.ATV.0000160551.21962.a7
29. Escobar
E. Hypertention and coronary heart disease (2002) J Hum Hypertens 16: S61-S63. https://doi.org/10.1038/sj.jhh.1001345
30. Viera
AJ, Hinderliter AL, Kshirsagar AV, Fine J and Dominik R. Reproducibility of
masked hypertension in adults with untreated borderline office blood pressure:
comparison of ambulatory and home monitoring (2010) Am J Hypertens 23: 1190-1197. https://doi.org/10.1038/ajh.2010.158
31. Watanabe
S, Kang DH, Feng L, Nakagawa T, Kanellis J, et al. Uric acid, hominoid
evolution, and the pathogenesis of salt-sensitivity (2002) Hypertension 40:
355-360. https://doi.org/10.1161/01.hyp.0000028589.66335.aa
32. Mazzali
M, Hughes J, Kim YG, Jefferson JA, Kang DH, et al. Elevated uric acid increases
blood pressure in the rat by a novel crystal-independent mechanism (2001)
Hypertension 38: 1101-1106.
https://doi.org/10.1161/hy1101.092839
33. Lin
C, Zhang Pu, Xue Y, Huang
Y and Ji K. Link of renal microcirculatory dysfunction to increased coronary
microcirculatory resistance in hypertensive patients (2017) Cardiol J 24:
623-632. https://doi.org/10.5603/CJ.a2017.0074
34. Sánchez-Lozada
LG, Tapia E, Santamaría J, Avila-Casado C, Soto V, et al. Mild hyperuricemia
induces vasoconstriction and maintains glomerular hypertension in normal and
remnant kidney rats (2005) Kidney Int 67: 237-247. https://doi.org/10.1111/j.1523-1755.2005.00074.x
35. Chae
JS, Kim OY, Paik JK, Kang R, Seo WJ, et al. Association of Lp-PLA(2) activity
and LDL size with interleukin-6, an inflammatory cytokine and oxidized LDL, a
marker of oxidative stress, in women with metabolic syndrome (2011)
Atherosclerosis 218: 499-506. https://doi.org/10.1016/j.atherosclerosis.2011.06.036. 36. Schroder
K, Zhou R and Tschopp J. The NLRP3 Inflammasome: A Sensor for Metabolic Danger?
(2010) Science 327: 296-300. https://doi.org/10.1126/science.1184003
37. Maruhashi
T, Hisatome I, Kihara Y and Higashi Y. Hyperuricemia and endothelial function:
From molecular background to clinical perspectives (2018) Atherosclerosis 278:
226-231. https://doi.org/10.1016/j.atherosclerosis.2018.10.007
38. Theilmeier
G, De Geest B, Van Veldhoven PP, Stengel D, Michiels C, et al. HDL-associated
PAF-AH reduces endothelial adhesiveness in apoE-/- mice (2000) FASEB J 14:
2032-2039. https://doi.org/10.1096/fj.99-1029com
39. Britesa
F, Martina M, Guillasb I and Kontush A. Antioxidative activity of high-density
lipoprotein (HDL): Mechanistic insights into potential clinical benefit (2017)
BBA Clinical 19: 66-77. https://doi.org/10.1016/j.bbacli.2017.07.002
Gao Yan, Shekou
Peoples Hospital, Shenzhen, Guangdong 518067, China, Tel: 86-0755-21606999, Fax: 86-0755-26889432, Email: gaoyan_0222@163.com Yan G, Ling L, Fang Z, Yuan C and
Ying C. Effect of increasing the plasma phospholipase A2 mass on the risk of
masked hypertension in humans (2019) Biochem Modern Appl 2:
63-68. Biomarker, Vascular inflammation, Oxidative
stress, Endothelial dysfunction, Blood pressure variabilityEffect of Increasing the Plasma Phospholipase A2 Mass on the Risk of Masked Hypertension in Humans
Abstract
Full-Text
Introduction
Methods
Ambulatory
Blood Pressure Measurements (ABPM)
Measurement
of Lp-PLA2
Other
Measurements
Diagnostic
and Inclusion Criteria
The
Exclusion Criteria Were As Follows
Results
Discussion
Conclusion
References
Corresponding author
Citation
Keywords