Introduction
The
premature birth affects 10% of deliveries; thanks to the advanced assistance
technologies, the survival, even at the GA (22-23 weeks) considered unthinkable
in the past, is now possible. Premature infants are at high risk of serious
complications such as NEC. It is one of the most dangerous pathologies linked
to prematurity because it is encumbered with a mortality ranging from 10 to 50%
[1]. Currently, the feeding with the breast milk is referred to as one of the
most important and effective prevention strategies of NEC. A protective effect
of the mothers milk has also been demonstrated against other complications of
prematurity such as retinopathy of prematurity, broncho-pulmonary dysplasia and
sepsis. Equally important are the beneficial effects that the breast milk can
have on the quality of neurological development of the preterm infant [2-21]. Several
studies show a direct relationship between the quantity and the duration of the
feeding with the breast milk and the neurological performance, even some years
later. This is particularly relevant for this category of children who are at
high risk of negative neurological outcomes. The use of the OMM improves the
food tolerance and reduces the duration of the parenteral nutrition by favoring
the fastest achievement of exclusive enteral feeding and reducing the risk of
infections related to the use of central venous catheters. Structured promotion
of breast milk expression is associated with shortened hospitalization for very
preterm infants [21-25].
These
effects of the breast milk are attributable to the wealth of bioactive
components, highlighted by the new technologies in the biological field, including
stem cells. These factors favor the development and the maturation of organs
and systems, first of all the intestines. Thats the reason why the breast milk
is considered the best choice for the premature baby and is often referred to
as an essential drug [26-38]. When the mothers milk is not available, the
alternative is represented by the human milk devoted to the banks by generous
donors. In Italy there are currently 38 milk banks coordinated by Italian
Association of Milk Banks (AIBLUD). The treatment of the DM partly reduces the
biological heritage of the mothers milk and also the macronutrient composition.
However, clinical practices demonstrate that many beneficial properties of DM still
persist after treatment [39-44].
OMM
and even more DM have a protein and caloric content that is not adequate to
support the growth that should be similar to the intrauterine growth,
especially the brain that is very sensitive to nutritional deficiencies during the
last trimester of pregnancy. For this reason human milk must be adequately
fortified. In particular increased protein intake may provide short and long
term benefits in terms of growth and neurodevelopment in human milk-fed ELBW
infants [45-49]. In the NICU of Casa Sollievo della Sofferenza, for premature
babies weighing less than 1800 grams only the HM is used, OMM or DM from
Allattiamolavita, the HMB operating for 10 years. HM is fortified when the
enteral volume of 60ml/kg is reached. The individualized fortification, based
on the evaluation of the macronutrient content of human milk (the human milk
analyser (HMA, Miris AB®, Uppsala, Sweden), is used.
Objectives
· Evaluation
of the food tolerance and of the incidence of NEC on premature infants of GA≤32
weeks fed during the NICU hospitalization exclusively with fortified HM.
· Evaluation
of any statistical significance in the comparison between the two kinds of
feeding, with OMM and EDM in the frame of symptoms related to the food
tolerance.
· Assessment of possible statistical significance regarding the use of OMM and/or DM with respect to GA and BW.
Materials and Methods
This
prospective analysis has been performed in the period from January 2016 to June
2017 on a sample of 48 premature infants with GA≤32 weeks. Infants with
congenital malformations or connatal infections were not enrolled. All donors
signed a written informed consent to donate their milk to clinical or research
use therefore the ethics committee of Authors Institution ruled that no formal
ethics approval was required in this particular case. The milk is administered
every 2hours for newborns weighing <1250 grams and every 3 hours for those
of a higher weight. We identified 4 groups-newborns fed with Exclusively Own
Mother Milk (EOMM), newborns who received Prevalent Own Mother Milk (POMM),
newborns who received Exclusively Donor Milk (EDM) and newborns who received
Prevalent Donor Milk (PDM). The evaluation of the following parameters was
performed in relation to the food tolerance-Gastric Residual (food/bilious)
(GR), Vomit (food/bilious) (V) and days of Feeding Suspension (FS). The
collected data have entered in a specific database.
Additional group comparisons were carried out between newborns who received EMOM or PMOM and newborns who received EDM or PDM using two-sample un-paired t-test. Continuous variables were reported as mean ± standard deviation while categorical variables were reported as frequency and percentages. A p-value <0.05 was considered as statistical significant. All analyses were performed using SAS (SAS institute, Cary, NC, USA). A further evaluation concerned the number of newborns fed with different modalities- OMM for the duration of the stay in NICU, OMM for the first days of life plus DM to cover the entire demand, only DM, and finally, the total amount of DM used to feed these preterm infants before the availability of their mothers milk or in its absence (Tables 1, 2, 3 and 4). We didnt analyze the feeding with preterm formula because this kind of milk is used only for infants in discharge fed with DM and it is not ethically possible to steal the milk from mothers or from the bank for the sole purpose of carrying out a study. The results have been divided into population data and tolerance data.
Results
Data on Population
The
population includes 48 preterm infants of GA ≤ 32 weeks-theres one case of a
high preterm delivery of 23 weeks, 3 of 24 weeks, 2 of 25 weeks, 5 of 26 weeks,
6 of 27 weeks, 3 of 28 weeks, 4 of 29 weeks, 8 of 30 weeks, 10 of 31 weeks and
7 of 32 weeks. Regarding the BW, the study sample includes 15 newborns weighing
<1000grams, 27 newborns with BW between 1000 gr and 2000 grams and 5
newborns weighing >2000grams. 29 are the VLBW infants (BW<1500grams)
(Figure 1,
Table 3). The period of hospitalization is proportionate to the GA and to the
BW with an average of 73.75 days (min 33, max 93) for the newborns weighing
<1000 grams, of 42.96 days for those having a wide range and weighing
between 1000 and 2000 grams (min 16, max 113) and of 21.25 days for those born
weighing >2000grams (18-29 days). Regarding the GA, the average is 39 days
(min 18, max 113) for those born between 28 and 32 weeks of GA, and 71.7 days
(min 27, max 109) for infants between 23 and 27 weeks.
Distinguishing the categories of infants according to the feeding, 5 preterm were fed with EOMM (including 3 VLBW), 17 newborns were fed with POMM (including 10 VLBW and 6 ELBW), 12 newborns received EDM (6 VLBW and 4 ELBW) and 14 received PDM (10 VLBW and 4 ELBW). 22 (44%) preterm infants were fed with EOMM or POMM and 26 (52%) with EDM o PDM. For the category of ELBW, 6 newborns on 15 (40%) weighing <1000grams were fed with OMM (exclusive or predominant). The amount of OMM used was 19271 ml for 22 children (44% of the population), while the amount of DM was 16414 ml (52% of the population) (Figure 1 and Table 1).
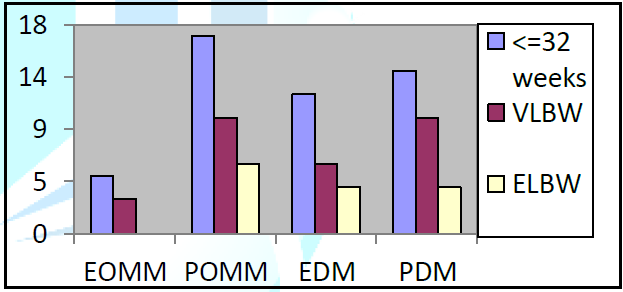
Data on Tolerance
39
(81.2%) newborns presented GR; 15 of them (38.4%) suffered for a number of days
<5, 8 of them (20%), between 6 and 15 days and the remaining 25 (64%) >15
episodes with a maximum of 36 (a 650grams patient hospitalized for 109 days).
In total, 457 events have been recorded, corresponding to 18% of days of
hospitalization (2464); bilious GR characterized 100 days corresponding to
21.8% of the total case of the GRs and 4% of the total events (Tables 1,2,3 and 4).
The
highest number of episodes concerned ELBW with 322 episodes, hence 70.4% of the
total; the number of V, related to 27 infants was 111 (56.2%) and 20 of them
(74%) reported a number of events <5, after 3 of them between 5 and 10, and
2 of them with 13 events. If we compare the tolerance to the kind of milk, 21
newborns (43.8%) reporting the gastrointestinal symptoms were fed with the DM-11
of them (22.9%) with EDM, 10 (20.8%) with PDM; 15 newborns (31.25%) were fed with
OMM, 3 of them (6.25%) with EOMM and 12 (25%) with POMM.
The
number of GRs was 289 (63.2%) for those fed with the DM- 178 (39.9%) (range
1-31) received EDM and 111 (24.28%) (range 1-20) PDM. The total for those fed
with OMM was 160 (35%)-12 (2.6%) EOMM and 148 (32.3%) with POMM. Regarding the
symptom of V, 15 newborns (31.25%) fed with DM were reported-9 of them (18.74%)
(range 1-13) EDM and 6 (12.5%) PDM. Moreover, 12 newborns fed with OMM are to
be distinguished into 4 (8.33%) with EOMM and 8 (16.6%) with POMM. 60 events of
Vs (53.56%) occurred among those fed with DM-37 of them (33%) (1-13) with EDM
and 23 (20.5%) (range 1-13) with PDM. The other 58 events of V (51.57%) with
OMM concerned 26 (23.2%) (range 1-5) with EOMM and 32 (28.57%) (1-5) with POMM.
If
we relate this data to the suspension of feeding, on the basis of the internal
NICU guidelines, the total days of FS during the hospitalization for the entire
population is 96 out of 2464 (3.8%) and involves 17 newborns (35%)-in detail, the
suspension lasted 1 day for 2 newborns, 2 days for other 6, 4 days for 2 of them,
6/8 days for 3, 2 weeks for 2 newborns, and in one case 20 days. According to the
kind of feeding, 56 days of FS have been recorded-58.3% of the FS occurred
among those fed with DM, 33 of them (34.3%) (range 1-7) were fed with EDM and
23 (23,9%) (range 2-14) with PDM. No day of FS has been recorded for those fed
with EOMM and 29 days (30.2%) (range 2-22) for those fed with POMM.
If
we concentrate on the VLBW (<1500grams), on a total of 338, 15 of them
(48.38%) reported the symptoms and were fed with DM- 7 (22.5%) EDM and 8
(25.8%) PDM. Among those fed with OMM, 13 cases occurred-3 (9.67%) with EOMM
and 10 (32.2%) with POMM. Regarding the number of GR, 179 were detected among
newborns who received DM-102 (30.1%) with EDM and 77 (22.7%) with PDM. Among those
who received mothers milk, 159 cases (47%) were highlighted-12 (3.5%) with EOMM
and 147 (43.49%) with POMM. Concerning the vomit, we recorded 60 daily
observations for those fed with DM-23 (27%) with EDM and 31 (35.6%) with PDM. The
cases of those who received OMM, were 26 (30.5%)-in detail, 12 (14, 1%) were
fed with EOMM and 14 (16.4%) with POMM. The days of suspension were 60 (68.9%
of the total) for newborns who received DM-29 (33.3%) with EDM and 31 (35.6%)
with PDM. For those fed with EOMM, there were no days of suspension; instead,
27 days (31%) have been registered where OMM was prevalent.
In
the population of 48 premature newborns of GA ≤32 weeks fed only with human
milk-
·
There
were no cases of NEC,
·
The
overall incidence of grs was 18%,
·
The
incidence of bilious residuals was 4%,
·
The
overall incidence of Vs. was 4.5%,
·
There
was no indication of blood in the stool,
·
The
total percentage of days of FS was 12.7%,
·
There
are differences between those fed with EOMM/POMM or EDM/PDM-
·
The
incidence of gastrointestinal events resulted very low in The group of Those
fed EOMM (2.6% GRs, 8.3% V) and There was no day of suspension of The enteral
feeding (Table 1,2,3),
· There
was no statistical correlation between The incidence of GR and V, The number of
days of feeding suspension and The duration of The hospitalization between The
two categories (OMM/DM),
· The correlation between the kind of feeding and the GA or the BW was not statistically significant (Table 4).
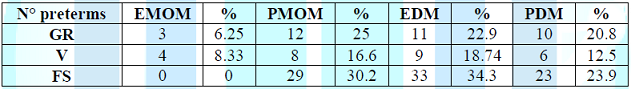
Table 1: Distribution of symptoms based on the
type of milk (number of preterm infants).
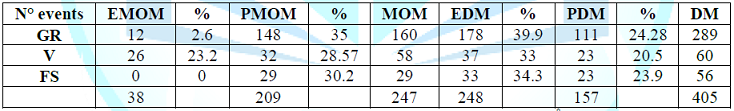
Table 2: Distribution of symptoms based on the
type of milk (N° events).
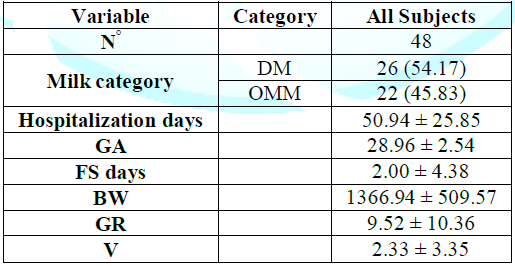
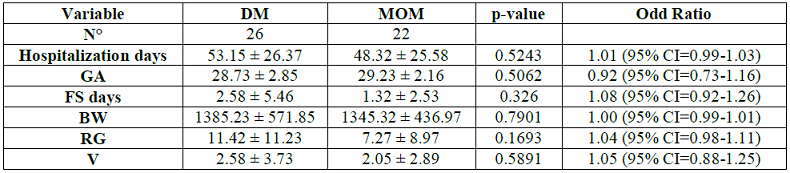
Table 4: Comparison of The variables.
Analysis of The results and discussion
The
first significant datum is the absence of serious events like NEC within the
population of preterm infants who were fed exclusively with HM. This is a very
important result which is not limited to the population recruited in this
study, but confirms the trend of zeroing the incidence of NEC for VLBW and has
been already published by the team of the milk bank of Casa Sollievo della
Sofferenza. This is validating that the exclusive use of HM is strongly
protective against the NEC. This is well understood if we think about the
innumerable bioactive factors composing the OMM, some of which only recently
discovered, that bring a defensive action against the inflammation, in addition
to exercising a trophic function on the immature intestine favoring maturation
and development [26-38,50].
Regarding
the intestinal tolerance, the numbers are comforting if we think that the
bilious GR and the vomits, which together with other suspect symptoms related
to abdominal pathologies (important abdominal distension, blood in the stool)
indicate the suspension of enteral feeding, are observed in a percentage
<5%. The highest incidence of GR (14%), especially if not bilious, is not
symptomatic of food intolerance and its not an early sign of NEC but of a
slower gastric emptying. This is a sign of immaturity that would explain the
prevalent share of GRs (about 70%) in the VLBW category [51,52]. In a paper, preterm
and LBW infants, feeding with formula compared with donor breast milk, either
as a supplement to maternal expressed breast milk or as a sole diet, result in
higher risk of developing NEC. Even in our study, the comparison clearly shows that
OMM is a life-saving drug. In effect, the exclusive use of OMM in our sample
led to the absence and to the minimal incidence of eating disorders and to the
non-suspension of feeding. This latest is a fundamental parameter of the
assistance-the quick achievement of the exclusive enteral feeding, the
subsequent suspension of the parenteral nutrition results in an important
reduction of the risk of complications and in a shorter duration of the
hospitalization [2-18,24,25,53].
The low incidence of GR during the exclusive feeding with OMM could be explained with a positive action on the gastrointestinal motility, as well as on the overall maturation including the digestion. It should be highlighted the role of the health personnel in encouraging the breast-feeding of preterm infants, reminding mothers of the importance of their milk, and monitoring from the first hours of birth the activation and compliance of the protocol inherent the breast-expressing, since it has proved to be an important strategy for milk production. The psychological support must be taken into account since pulling milk is a way for mothers to overcome the detachment and the psychological drama of a premature birth [54-56]. The comparison between the different kinds of feeding with HM demonstrates that the effect of maximum protection is in part reduced with the DM but remains suitable in improving the tolerance. the lower protective value of the DM is related to its treatment, predominantly involving the pasteurization and the freezing which inactivate or lose part of the immunological heritage and of the enzymes of OMM but overall, many of the positive effects, especially on tolerance, remain [30, 39-41,47-57,66].
The
oligosaccharides present in human milk (resistant to pasteurization) have a protective
effect from NEC having been shown an inverse correlation between their
concentration in milk and the risk of developing this very serious complication,
confirming the beneficial action already detected in rats [58,59]. A new
pasteurization method is being assessed-a rapid 52-degree process of 5-15
seconds to be used by milk banks that ensures a better preservation of the
biological factors of the OMM and that probably will reduce the differences
between DM and OMM [60-62].
The
precise indication given by the scientific societies and the institutions
dealing with child health is to use the DM as alternative to OMM, which remains
at the first place in the hierarchy of choices. However, the result of the
statistical comparison between the two kinds of feeding in which no
significance was found, confirms their validity. The availability of DM depends
on the generosity of the donors who, in a spontaneous and gratuitous way,
devote the precious OMM to the Human Milk Banks (HMB). Currently, it is
estimated that in Italy the quantity of milk collected in The 38 milk banks and
distributed throughout the national territory, can cover only 30% of the needs
of the NICUs [63].
The
absence of statistical significance between the OMM and the DM in relation to
GA and BW shows that even infants of VLBW or ELBW can be fed with their mothers
milk. Moreover, in the study population, 60% of VLBW have received OMM. Several
stories, even extraordinary, regarded mothers of highly preterm infants inside the
BLUD Allattiamolavita who succeeded in feeding their children and in donating a
part of their milk to the bank [64,65]. The study confirms the importance of the
presence of the milk bank as an integral part of the NICU, which worked as
supplier of donated HM and as facilitator for the milk production. 6 preterm on
15 (40%) having a BW less than 1000 grams, were fed with their mothers milk, though
not always exclusively. We retain that this result deserves attention [66-68].After
all, the ultimate aim of this work is to improve the feeding of preterm
infants-an aspect considered fundamental in the care of newborns due to the
possible effects on mortality and morbidity in the short and long term
[12,9-21,23].
Conclusions
The study highlights the irreplaceability of the OMM in feeding the premature baby, especially if VLBW-it is a real essential drug that helps preventing NEC and food intolerance. It also demonstrates that the DM is a valid substitute if taken before OMM or in case of lack, because it holds the protective capacity against the NEC and the feeding tolerance is not statistically different compared to the OMM. The support to the milk production is interlinked to the donation; the presence of a NICU milk bank promotes the availability of the mothers milk for preterm infants. Only the dissemination of the culture about the breastfeeding and the milk donation, with the implementation of effective protocols, can lengthen the use of HM, which is a guarantee of better health and well-being for the present and the future of this fragile category of newborns.Acknowledgment
We thank all those who directly and indirectly
took part in the work- those who, every day, take care of the feeding of our
premature babies, those who collected the data step by step, those who
contributed to processing them and the scientific director of Casa Sollievo
della Sofferenza who approved and consented to the study and its publication.
References
1. Sharma R and Hudak ML. A clinical perspective of necrotizing enterocolitis-past, present, and future (2013) Clin Perinatol 40-27-51. https://doi.org/10.1016/j.clp.2012.12.012
2. Maffei D and Schanler RJ. Human milk is the feeding strategy to prevent necrotizing enterocolitis (2017) Semin Perinatol 41: 36-40. https://doi.org/10.1053/j.semperi.2016.09.016
3. Johnson TJ, Patel AL, Bigger HR, Engstrom JL and Meier PP. Cost savings of human milk as a strategy to reduce the incidence of necrotizing enterocolitis in very low birth weight infants. (2015) Neonatology 107: 271-276. https://doi.org/10.1159/000370058
4. Hair AB, Peluso AM, HawThorne KM, Perez J, SmiTh DP, et al. Beyond necrotizing enterocolitis prevention-improving outcomes with an exclusive human milk-based diet (2016) Breastfeed Med 11: 70-74. https://doi.org/10.1089/bfm.2015.0134
5.Buckle A and Taylor C. Cost and cost-effectiveness of donor human milk to prevent necrotizing enterocolitis-systematic review (2017) Breastfeed Med 12: 528-536 https://doi.org/10.1089/bfm.2017.0057
6. Herrmann K and Carroll K. An exclusively human milk diet reduces necrotizing enterocolitis (2014) Breastfeed Med 9: 184-190. https://doi.org/10.1089/bfm.2013.0121
7.Patel AL and Kim JH. Human milk and necrotizing enterocolitis (2018) Semin Pediatr Surg 27: 34-38. https://doi.org/10.1053/j.sempedsurg.2017.11.007
8. Kantorowska A, Wei JC, Cohen RS, Lawrence RA, Gould JB, et al. Impact of donor milk availability on breast milk use and necrotizing enterocolitis rate (2016) Pediatrics 137: 1-3. https://doi.org/10.1542/peds.2016-0784
9.Meinzen-Derr J, Poindexter B, Wrage L, Morrow A L, Stoll B, et al. Role of human milk in extremely low birth weight infants risk of necrotizing enterocolitis or death (2009) J Perinatol 29: 57-62. https://doi.org/10.1038/jp.2008.117
10.Rozé JC, Ancel PY, Lepage P, Martin-Marchand L, Al Nabhani Z, et al. Nutrition epipage 2 study group; epiflore study group. nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants (2017) Am J Clin Nutr 106: 821-830. https://doi.org/10.3945/ajcn.117.152967
11. Caplan MS. Paediatrics-Are human milk oligosaccharides the magic bullet for necrotizing enterocolitis? (2017) Nat Rev Gastroenterol Hepatol 14: 394-395. https://doi.org/10.1038/nrgastro.2017.65
12.Chowning R, Radmacher P, Lewis S, Serke L, Pettit N, et al. A retrospective analysis of the effect of human milk on prevention of necrotizing enterocolitis and postnatal growth (2016) J Perinatol 36: 221-224. https://doi.org/10.1038/jp.2015.179
13.Cortez J, Makker K, Kraemer DF, Neu J, Sharma R, et al. Maternal milk feedings reduce sepsis, necrotizing enterocolitis and improve outcomes of premature infants (2017) J Perinatol 38: 71-74. https://doi.org/10.1038/jp.2017.149
14.Bharwani SK, Green BF, Pezzullo JC, Bharwani SS, Dhanireddy R, et al. Systematic review and meta-analysis of human milk intake and retinopathy of prematurity: a significant update (2016) J Perinatol 36: 913-920. https://doi.org/10.1038/jp.2016.98
15.Zhou J, Shukla VV, John D and Chen C. Human milk feeding as a protective factor for retinopathy of prematurity: a meta-analysis (2015) Pediatrics 136: e1576-e1586. https://doi.org/10.1542/peds.2015-2372
16. Dicky O, Ehlinger V, Montjaux N, Gremmo-Féger G, Sizun J, et al. Epipage 2 nutrition study group; epinutri study group.policy of feeding very preterm infants with their mothers own fresh expressed milk was associated with a reduced risk of bronchopulmonary dysplasia (2017) Acta Paediatr 106: 755-762. https://doi.org/10.1111/apa.13757
17. Patel AL, Johnson TJ, Robin B, Bigger HR, Buchanan A, et al. Influence of own mothers milk on bronchopulmonary dysplasia and costs (2017) Arch Dis Child Fetal Neonatal Ed 102: F256-F261. http://dx.doi.org/10.1136/archdischild-2016-310898
18. Horta BL, Loret de Mola C and Victora CG. Breastfeeding and intelligence-a systematic review and meta-analysis (2015) Acta Paediatr 104: 14-19. https://doi.org/10.1111/apa.13139
19. Schanler RJ. Outcomes of human milk-fed premature infants (2011) Semin Perinatol 35: 29-33. https://doi.org/10.1053/j.semperi.2010.10.005
20. Park S, Kim BN, Kim JW, Shin MS, Yoo HJ et al. Protective effect of breastfeeding with regard to childrens behavioral and cognitive problems (2014) Cho SC Nutr J 13. https://doi.org/10.1186/1475-2891-13-111
21. Madore LS, Bora S, Erdei C, Jumani T, Dengos AR, et al. Effects of donor breast milk feeding on growth and early neurodevelopmental outcomes in preterm infants-an observational study (2017) Clin Ther 39: 1210-1220. https://doi.org/10.1016/j.clinthera.2017.05.341
22. Klingenberg C, Muraas FK, Isaksen CE, Nilsen T, Torgersen M, et al. Growth and neurodevelopment in very preterm infants receiving a high enteral volume-feeding regimen-a population-based cohort study (2017) J Matern Fetal Neonatal Med 17: 1664-1672. https://doi.org/10.1080/14767058.2017.1414796
23. Sammallahti S, Kajantie E, Matinolli HM, Pyhälä R, Lahti J, et al. Nutrition after preterm birth and adult neurocognitive outcomes (2017) Plos one 12: https://doi.org/10.1371/journal.pone.0185632
24. Assad M, Elliott MJ and Abraham JH. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet (2016) J Perinatol 36: 216-220. https://doi.org/10.1038/jp.2015.168
25.Healy DB, Brennan AM, ODonovan R, Daly V, Doolan A, et al. Structured promotion of breastmilk expression is associated with shortened hospitalisation for very preterm infants (2016) Acta Paediatr 105: e252-e256. https://doi.org/10.1111/apa.13399
26. Fanos V, Reali A and Bardanzellu F. Omics in human colostrum and mature milk- looking to old data with new eyes (2017) Nutrients 9: 1-24. https://doi.org/10.3390/nu9080843
Preterm feeding, Donor human milk, Food
tolerance.


 PDF
PDF