Clinical Presentation
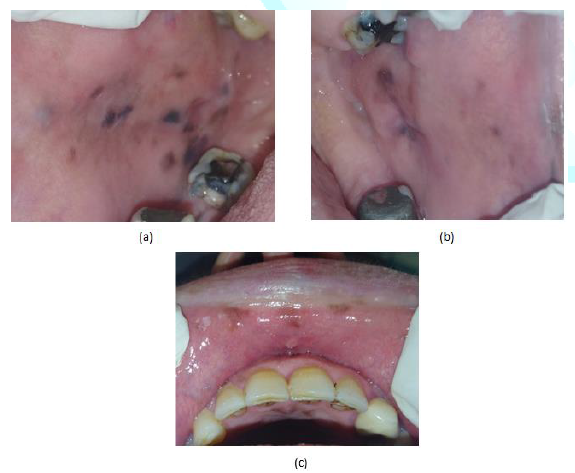
A previously healthy 58-year-old female patient was presented for a routine dental examination. On clinical examination asymptomatic multifocal brownish macules ranging from 3 to 6 mm were found on the right and left buccal mucosae and the upper labial mucosa (Figure 1). Extra-oral examination revealed non-palpable sub-mandibular, sub-mental and cervical lymph nodes as well as a normal peri-oral skin and light brown complexion. The comprehensive medical history revealed a recent history of nausea, fatigue, and decreased appetite during the preceding month. The patient was unaware of the onset of the discovered pigmentations. The vital signs were measured revealing normal temperature, blood pressure, respiratory rate, and pulse. The patient was a non-smoker and a non-alcoholic.
Differential Diagnosis of Oral Pigmented
Lesions
The differential
diagnosis of oral pigmentation includes a wide range of both focal and
multifocal or diffuse pigmentation. Most of the oral pigmented lesions are
benign and require simple assurance. However, malignant pigmented lesions such
as malignant
melanoma and Kaposi's sarcoma could be encountered. In
addition, oral pigmentation could be a sign of an underlying systemic disease [1-3].
In order to
evaluate a particular oral pigmented
lesion a comprehensive dental and medical history should
be taken from the patient as well as extraoral and intraoral examinations. In
some cases, laboratory investigations could be useful to confirm the diagnosis.
The medical history should include the onset, and Location of the lesion, any
associated constitutional symptoms such as fatigue, malaise, anorexia, weight
loss), drug intake, and habits such as smoking and alcohol consumption. The
patient should be asked for any extra oral skin or mucosal pigmentations,
particularly, in the peri-oral region and the genitalia. The intraoral
examination includes an assessment of the distribution (i.e. unilateral or
bilateral), pattern (i.e. focal, multifocal or diffuse), size, and color of the
lesion. If the lesion was blood-filled, diascopy test is used to determine
whether it was intravascular or extravascular. In this paper we propose an
algorithm (Figure 2) to be followed
for the assessment of most oral pigmented lesions based on the history and the
clinical presentation.
Focal Pigmented Lesions
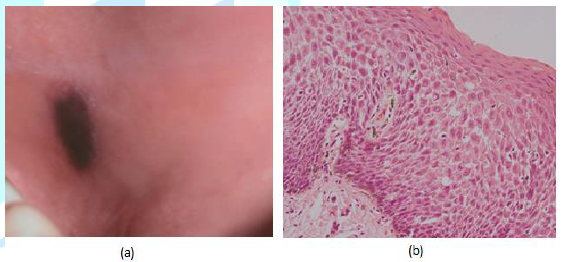
Oral/
labial melanotic macule is the most common oral melanotic
lesions. It usually develops on the lower lip (labial
melanotic macule) and the gingiva. It can also occur elsewhere in the oral
mucosa. In most cases, melanotic macules are small (<1 cm) and well defined
which does not enlarge, once reached a specific size. Microscopically,
melanotic macules show an abundance of melanin pigment in the basal cell layer,
which is often, accentuated at the tips of the rete pegs. It is not associated
with melanocyte proliferation. Thus, it is totally benign and is not known to
transform into malignant melanoma (Figure
3) [4-7].
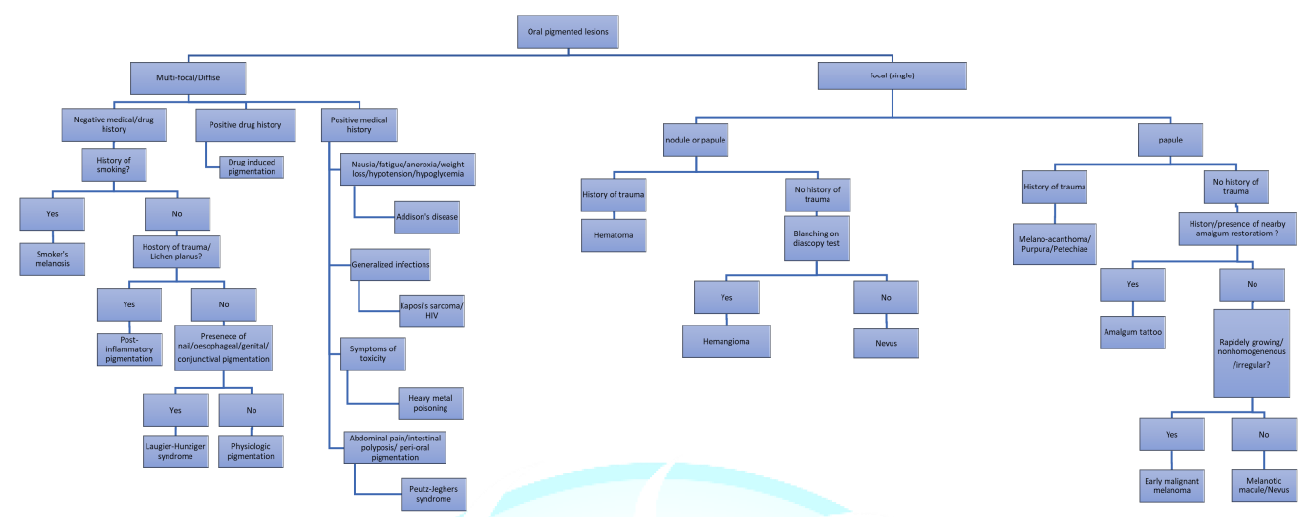
Figure2: An algorithm for the evaluation of pigmented lesions of the oral cavity.
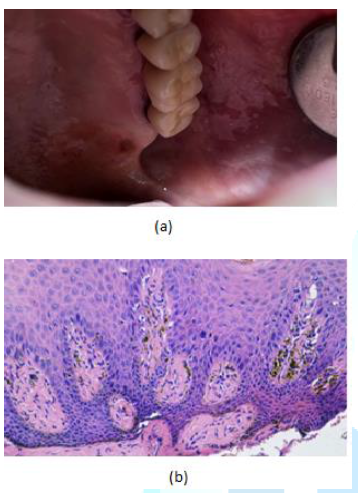
Melanocytic nevus is a rare cause of focal oral pigmentation. It can be classified into junctional, compound, intramucosal, and blue nevi. Junctional nevi presents mostly as a brown to black macule while compound and intramucosal nevi might be lighter in color, dome-shaped, and most commonly seen in the buccal mucosa. Blue nevi develop deep in the connective tissue which may account for its color and develop most commonly in the palate. Histologically melanocytic nevi develop from the benign proliferation of melanocytes. They are believed to have precursor cells that may transform into malignant melanoma. For this reason, nevi, particularly, in the palate should be biopsied (Figure 4) [4,8-10].
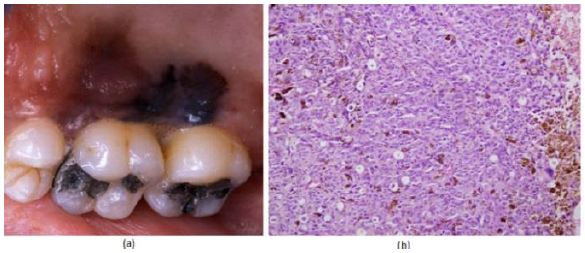
Amalgam
tattoo
is the most common pigmented lesion in the oral cavity. It appears as a
grey-blue macule most commonly on the gingiva, alveolar mucosa, and buccal
mucosa. Amalgam tattoo may be introduced through a variety of dental procedures
by which amalgam particles can be carried into a laceration or an extraction socket
or even intact mucosa. Diagnosis could be made on the basis of radiographic
examination when the amalgam particles are large enough. In this case, fine
radio-opacities could be observed. If not possible and it is in a suspicious
location such as the hard palate, taking a biopsy is advised to rule out
malignant neoplasm. Under the microscope, fine black granules or fibrils are
seen embedded in the connective tissue with little or no inflammatory reaction
(Figure 5) [2,4,5,11,12].
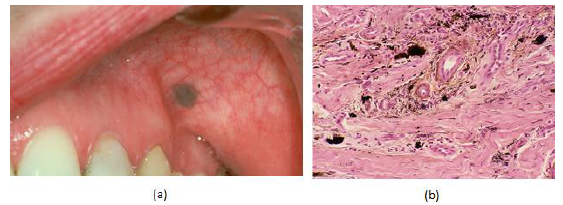
Oral
melanoacanthoma is a reactive benign pigmented lesion that usually
has an acute onset in response to trauma or local irritation, frequently in
dark skin population. Clinically, it is brownish in color, has ill-defined margins,
and enlarges rapidly. Thus, a biopsy is mandatory to exclude malignancy. The
most common site of oral
melanoacanthoma is the buccal mucosa. On microscopic
examination, it shows the proliferation of the benign dendritic melanocytes in
the acanthotic and the spongiotic layers of the epithelium (Figure 6) [4,8,13,14].
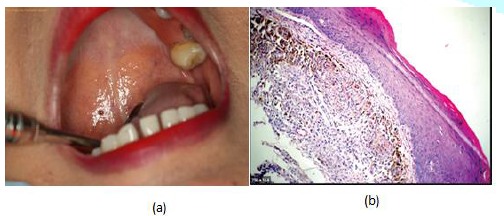
Hemangioma is the proliferation of vascular endothelial cells. Hemangiomas occur to 4-5% of infants aging 1 year, making it the most common tumor of infancy. It is an elevated lesion whose color ranges from red to blue or purple. Diagnosis is often based on blanching with a diascopy test. This test is performed by applying gentle pressure on the lesion by using a glass slide. Blanching indicates an intra-vascular lesion (Figure 7) [2,15,16].
Varix is
defined as abnormally dilated veins observed in old individuals. This most
common site of varix is the ventral surface of the tongue where they appear as
small soft purplish granules that blanch on diascopy test (Figure 8) [2].
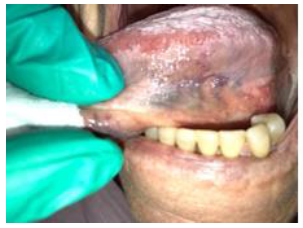
Figure8:Sublingual varix in 75-year old male.
Hematoma,
petechiae, purpurae, and ecchymoses are
extravascular lesions caused by the extrusion of blood into the connective
tissue following a traumatic event. The color of these lesions will appear red
initially and turns into brown gradually as a result of the degradation of
hemoglobin in the extravasated red blood cells into hemosiderin and usually
disappears within 2 weeks. The differentiation between these lesions is upon
clinical examination. Hematoma is an elevated lesion, while petechiae,
purpurae, and ecchymoses are flat and differ only in their sizes. Petechiae are
characterized by being pinpoint. The diameter of purpura ranges from 2 mm to 2
cm, while ecchymosis is larger than 2 cm in diameter (Figure 9) [2,4,16,17].

Figure9:Hematoma and petechias in a 26 year old patients with leukemia.
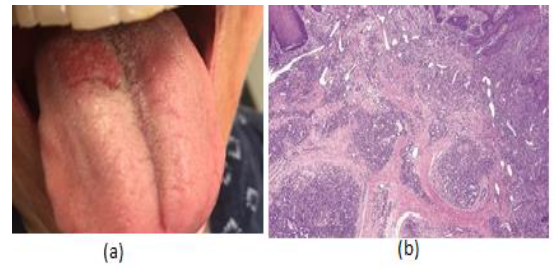
Malignant melanoma is a
rare oral cancer representing less than 1% of oral malignant tumors. It is a
malignant proliferation of melanocytes in the junction between the epithelium
and the connective tissue as well as in the connective tissue. The most common
site of oral melanoma is the hard palate followed by the maxillary gingivae
which together represent two thirds of the cases. Oral melanomas are usually
encountered between the fifth and the seventh decades of life and in
black-skinned individuals, who have 3 to 4 times more risk than whites. It
presents as a black or brown slowly-growing patch with a non-homogeneous color
and ill-defined borders or a rapidly-enlarging mass associated with bleeding
ulceration and pain. However, up to one third of the cases exhibit no
pigmentation (amelanotic) (Figure 10)
[2,4,8,10, 17-19].
Multi-Focal/Diffuse Pigmentation
Physiologic
(racial) pigmentation is the most common multi-focal or
diffuse oral pigmentation. It
is more common in dark-skinned individuals such as Africans, Asians, and
South-Americans. Physiologic pigmentation usually appears during the first two
decades of life, but may not be noticed by the patient until adulthood. The
attached gingiva is the most commonly affected site, although pigmentation of
the labial and buccal mucosae is not uncommon where it appears usually as ill-defined
patches. On the microscopic level, increased melanin pigment within the basal
cell layer can be observed. Physiologic pigmentation could be considered as a
differential diagnosis for the presented case, provided that the patient had a
brown complexion (Figure 11)
[2,4,8,20].
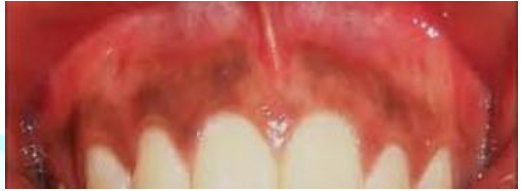
Figure11:Physiological pigmentation involving the gingiva.
Drug-induced
melanosis
is a common cause of multi-focal or diffuse oral pigmentation. A number of
drugs may cause this type of pigmentation including antimalarials such as
chloroquine, hydroxychloroquine, and quinacrine. These drugs are also used for
autoimmune diseases. Other examples of drugs related to oral melanosis are
minocycline, tetracycline, oral contraceptives, and cytotoxic medications such
as cyclophosphamide and busulfan. There are multiple mechanisms for drug-induced oral
pigmentation. According to the type of the drug
used, it may include the accumulation of melanin, the deposition if the drug
itself or one if its metabolites, a drug-induced synthesis of pigments such as
lipofuscin, or the deposition of iron due to vessels damage and red blood cells
lysis. In our presented case, the patient did not give a history of any drug
intake; thus, drug-induced melanosis was excluded from the differential
diagnosis (Figure 12) [4,17,20-23].

Smoker's
melanosis
occurs most commonly in the labial gingiva followed by the buccal mucosae and
the palate of smokers. Up to 21.5% of smokers demonstrate this type of
pigmentation with females being more affected than males. Smoker's melanosis
appears as flat brown map-like or geographic areas. In dark-skinned individuals
showing physiologic pigmentation, smoking may accentuate the oral pigmentation.
Despite caused by smoking, smoker's melanosis is not potentially malignant and
returns to normal after cessation of smoking. Since our case was a non-smoker,
this was not considered as a differential diagnosis as well (Figure 13) [4,8,24-27].
Post-inflammatory
(inflammatory) pigmentation appears simultaneously or following
injuries or long-standing
inflammatory diseases, particularly, lichen planus. Clinically,
diffuse brown pigmented areas are seen in proximity to reticular, erosive, or vesicular
lesions. This is more common in individuals with a dark complexion.
Histologically, there is increased basilar melanin and melanin laden macrophages
in the connective tissue. The clinical examination of the reported case did not
reveal any inflammatory disease underlying the pigmentation. Also there was no
history of injury or trauma (Figure 14) [2,8, 28].
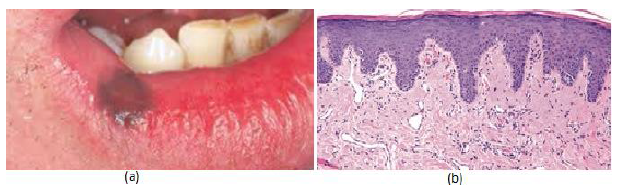
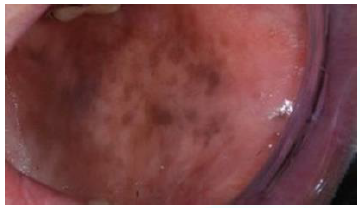
Figure14:Post inflammatory pigmentations in a patient with oral lichen planus.
Kaposi's
sarcoma
(could be solitary in the oral cavity) is a multi-focal vascular malignancy
that develops at the late stages of Human
Immunodeficiency Virus (HIV) disease and is considered the
most common neoplasm in AIDS patients. In one study 45% of AIDS patients had
oral Kaposi's sarcoma. Another study reported that 27 out of 53 AIDS-related
Kaposi's sarcoma occurred in the oral cavity. Early Kaposi's sarcoma appears as
red to brown macules or patches which are often bilateral. In the late stages
it becomes nodular, bleeding, and ulcerating. It usually occurs in the palate, gingiva,
or the tongue. Our patient did not have any sign of AIDS, therefore, Kaposi's
sarcoma was excluded (Figure 15) [2,17,18,29,30].
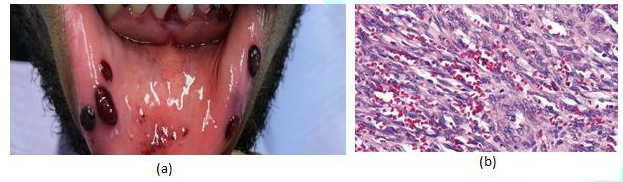
Peutz-Jeghers Syndrome is a
rare autosomal dominant disease associated with mutations in the LKB1/STK11
gene located on the short arm of chromosome. It is characterized by generalized
or multiple small brownish macules on the lower lip and in the perioral region.
Intranasal, conjunctival, and intraoral pigmentation can also be seen. Clinical
manifestations include intestinal polyposis and increased risk of
gastrointestinal cancer. This was not considered in our top-suspected diagnoses
due to the absence of any abdominal symptoms or perioral pigmentation and late
acute onset [8,19,31-33].
Laugier-Hunziker pigmentation is an idiopathic
pigmentation described as macular hyperpigmentation of the oral mucosa,
particularly, the labial and buccal mucosae. Other mucosal surfaces can also be
affected such as the esophageal, genital, conjunctival mucosae. In addition,
60% of patients exhibit nail pigmentation in the form of melanotic streaks.
Laugier-Hunziker pigmentation is diagnosed by exclusion when all other
potential causes of oral pigmentation are eliminated. For this reason, it was
considered as the last differential diagnosis, in case other suspected
diagnoses are excluded [4,34,35].
Addison's
disease or primary hypoadrenalism is caused by the progressive
destruction of the adrenal glands. Autoimmune disease is one of the most common
etiologies of this destruction, together with neoplasms, trauma, medications, infection
and iatrogenic causes. Although weakness, fatigue, depression are the most
common early presenting signs of the disease, mucocutaneous hyperpigmentation
may be one of the earliest signs or even the first sign of the disease. As the
levels of the adrenal cortex hormones decrease, Adrenocorticotrophic Hormone
(ACTH) is secreted from the anterior pituitary gland to stimulate steroids
secretion from the adrenal cortex. Concurrently, α-Melanocyte-Stimulating
Hormone (α-MSH) serum levels also increase resulting in a generalized tan-like
appearance as well as bilateral multi-focal oral pigmentation. The case
presented in this paper reported a history of nausea, fatigue, and reduced
appetite. These signs in addition to the bilateral multi-focal pattern of
pigmentation in the buccal mucosae made Addison's disease one
of our suspected differential diagnoses [2,4,35-38].
Given that the
presented oral pigmentation followed a bilateral multi-focal pattern, focal
pigmentations were excluded from our differential diagnosis. Therefore, our top
differential diagnoses were: 1) Physiologic pigmentation being the most common
diffuse or multi-focal pigmentation, 2) Pigmentation associated with Addison's
disease, given the systemic manifestations presented such as nausea, fatigue,
and reduced appetite and the unusual pattern of the pigmentation, 3)
Laugier-Hunziger pigmentation after excluding the two aforementioned diagnoses.
Laboratory Investigations and Diagnosis
Given that
pigmentation associated with Addison's disease was one of the proposed
diagnoses, laboratory investigations were requested. The requested tests were:
morning serum cortisol and blood chemistry. The morning serum cortisol level
was below the minimum reference range (4.1 µg/dL, reference: 5-25 µg/dl). Blood
chemistry as well revealed a serum sodium level (137.2 mmol/L) at the lower
border of the normal range (136-150 mmol/L) and a slight hyperchloremia (106.9
mmol/L, Reference: 95-105 mmol/L). These findings suggest early Addison's
disease diagnosis. The patient was referred to the endocrinology department for
further investigations and management.
Discussion
Addison described
the main features of her disease as:" general languor and debility,
remarkable feebleness of the heart’s action, irritability of the stomach, and a
peculiar change of color in the skin, occurring in connection with a diseased
condition of the suprarenal capsules". Primary adrenal insufficiency is
known as Addison's disease, results in the reduction or the lack of the
glucocorticoids (primarily cortisol) and mineralocorticoids (primarily
aldosterone). The symptoms associated with the disease are attributed to the
low cortisol and aldosterone levels. Reduced serum cortisol leads to
hypoglycemia, weakness, and fatigue, while low serum aldosterone causes an
electrolyte imbalance: decreased sodium and chloride and increased potassium.
This in turn results in hypotension, weight loss, and salt craving. Addison's
disease patients also present with anorexia, skin, and mucosal
hyperpigmentation. Hyperpigmentation in Addison's disease is a manifestation of
increased Adrenocorticotrophic Hormone (ACTH) and α-Melanocyte Stimulating
Hormone (α -MSH) from the pituitary gland as a feedback mechanism of adrenal
failure [4,39-42].
Hyperpigmentation
has been perceived as a hallmark sign of Addison's disease. Skin pigmentation
can be generalized presenting as a bronze or a tan-like appearance that is difficult
to detect in dark-skinned individuals. Besides, pigmentation can be more
prominent in the sun-exposed areas of the skin such as the face, the neck, and
the hands, also at the sites of friction, palmer creases, and recent scars. The
buccal mucosa, vermilion border of the lips, gingiva, and vaginal mucosa may
also exhibit areas of hyperpigmentation [37,39,40,43,44].
Evidence considered
mucocutaneous
pigmentation as a useful diagnostic aid for
Addison's disease. This is confirmed by multiple case reports which reported
mucocutaneous pigmentation as an early or the only sign of Addison's disease.
Hydar et al. [45] demonstrated that in six out of seven patients with adrenal
insufficiency presented with hyperpigmentation as the first symptom of the
disease. Najjar and Jarrah reported a case in which tan-like skin pigmentation
was the only sign of Addison's disease. Smith as well published a case report
demonstrating skin pigmentation as a diagnostic sign of the disease [4,35,43,45-47].
Although oral
pigmentation is not uncommon in Addison's disease, it can be misdiagnosed as
drug-induced melanosis, physiologic pigmentation, or other more common diffuse
or multi-focal intra-oral pigmentation. It has been reported as well that oral
pigmentation can be the sole manifestation of the disease and if diagnosed at
this early stage, this can prevent its progression and the associated
complications. Burk et al. [42] reported two cases of pigmentation in the
labial mucosa, tongue, and gingiva. After a thorough medical history, the two
cases were found to have systemic manifestations such as nausea, vomiting, and
fatigue. Laboratory tests confirmed their diagnosis as adrenal insufficiency.
Similarly, Lanza et al. [38] diagnosed a case with Addison's disease depending,
primarily, on oral pigmentation as a diagnostic sign. Moreover, tongue and lip
pigmentation were the only clinical signs for Addison's disease in a case
reported by Strakosch and Gordon [48].
Conclusion
Although the
diagnosis of Addison's disease before the development of systemic symptoms is
rare, it should be aimed for. Addison's disease should be considered as a
differential diagnosis for multi-focal and diffuse intra-oral pigmentation,
particularly when associated with constitutional and systemic symptoms such as
nausea, vomiting, and fatigue.
References
- Lenane
P and Powell F. Oral pigmentation (2002) JEADV 14: 448-465. https://doi.org/10.1046/j.1468-3083.2000.00143.x
- Adel
Kauzman B, Pavone M, Blanas N and Bradley G. Pigmented lesions of the oral
cavity: review, differential diagnosis, and case presentations (2004) J Can
Dent Assoc 70: 682-683.
- Cicek
Y and Ertas U. The normal and pathological pigmentation of oral mucous
membrane: a review (2003) J Contemp Dent Pract 4: 76-86. https://doi.org/10.5005/jcdp-4-3-76
- Burket
LW, Greenberg MS, Glick M and Ship JA. Burket's oral medicine (2008) BC Decker,
Canada
- Weathers
D, Corio RL, Crawford B, Giansanti J and Page L. The labial melanotic macule
(1976) Oral Surg Oral Med Oral Pathol 42: 196-205. https://doi.org/10.1016/0030-4220(76)90124-9
- Page
L, Corio R, Crawford B, Giansanti J and Weathers D. The oral melanotic macule
(1977) Oral Surg Oral Med Oral Pathol 44: 219-226. https://doi.org/10.1016/0030-4220(77)90272-9
- Gupta G, Williams R and Mackie R. The labial melanotic macule: a review of 79 cases (1997) Brit J Dermatol 136: 772-775.https://doi.org/10.1046/j.1365-2133.1997.6731621.x
- Gondak
RO, da Silva-Jorge R, Jorge J, Lopes MA and Vargas PA. Oral pigmented lesions:
Clinicopathologic features and review of the literature (2012) Med Oral Patol
Oral Cir Bucal 17: e919. https://doi.org/10.4317/medoral.17679
- Buchner
A and Hansen LS. Pigmented nevi of the oral mucosa: A clinicopathologic study
of 36 new cases and review of 155 cases from the literature: Part II: Analysis
of 191 cases (1987) Oral Surg Oral Med Oral Pathol 63: 676-682. https://doi.org/10.1016/0030-4220(87)90370-7
- Hicks
M and Flaitz C. Oral mucosal melanoma: epidemiology and pathobiology (2000)
Oral Oncol 36: 152-169. https://doi.org/10.1016/s1368-8375(99)00085-8
- Weathers
DR and Fine RM. Amalgam tattoo of oral mucosa (1974) Archi Dermatol 110:
727-728. https://doi.org/10.1001/archderm.110.5.727
- Müller
S. Melanin‐associated pigmented lesions of the oral mucosa: presentation,
differential diagnosis, and treatment (2010) Dermatol Therap 23: 220-229. https://doi.org/10.1111/j.1529-8019.2010.01319.x
- Goode
RK, Crawford BE, Callihan MD and Neville BW. Oral melanoacanthoma: review of
the literature and report of ten cases (1983) Oral Surg Oral Med Oral Pathol
56: 622-628. https://doi.org/10.1016/0030-4220(83)90080-4
- Fornatora
ML, Reich RF, Haber S, Solomon F and Freedman PD. Oral melanoacanthoma: a
report of 10 cases, review of the literature, and immunohistochemical analysis
for HMB-45 reactivity (2003) Ameri J Dermatopathol 25: 12-15. https://doi.org/10.1097/00000372-200302000-00003
- Neville
BW, Damm DD, Allen CM and Chi AC. Oral and maxillofacial pathol (2016)
Elsevier, United States 604-605.
- Carpenter
W and Rudd M. Focal, flat pigmentations of the oral mucosa: a clinical approach
to the differential diagnosis (2000) J Calif Dent Asso 28: 949-954.
- Hatch
CL. Pigmented lesions of the oral cavity (2005) Dent Clini 49: 185-201. https://doi.org/10.1016/j.cden.2004.07.013
- Eisen
D and Voorhees JJ. Oral melanoma and other pigmented lesions of the oral cavity
(1991) J Ameri Acad Dermatol 24: 527-537. https://doi.org/10.1016/0190-9622(91)70077-f
- Rapidis
AD, Apostolidis C, Vilos G and Valsamis S. Primary malignant melanoma of the
oral mucosa (2003) J Oral Maxillofa Surg 61: 1132-1139. https://doi.org/10.1016/s0278-2391(03)00670-0
- Eisen
D. Disorders of pigmentation in the oral cavity (2000) Clini Dermatol 18:
579-587. https://doi.org/10.1016/s0738-081x(00)00148-6
- Kleinegger
CL, Hammond HL and Finkelstein MW. Oral mucosal hyperpigmentation secondary to
antimalarial drug therapy (2000) Oral Surg Oral Med Oral Pathol Oral Radiol
Endodontol 90: 189-194. https://doi.org/10.1067/moe.2000.106340
- Cockings
JM and Savage NW. Minocycline and oral pigmentation (1998) Austra Dent J 43:
14-16. https://doi.org/10.1111/j.1834-7819.1998.tb00145.x
- Dereure
O. Drug-induced skin pigmentation (2001) Ameri J Clini Dermatol 2: 253-262. https://doi.org/10.2165/00128071-200102040-00006
- Hedin
CA. Smokers' melanosis: occurrence and localization in the attached gingiva
(1977) Archi Dermatol 113: 1533-1538. https://doi.org/10.1001/archderm.113.11.1533
- Hedin
CA and Axell T. Oral melanin pigmentation in 467 Thai and Malaysian people with
special emphasis on smoker's melanosis (1991) J Oral Pathol Med 20: 8-12. https://doi.org/10.1111/j.1600-0714.1991.tb00879.x
- Axeix
T and Hedin Ca. Epidemiologic study of excessive oral melanin pigmentation with
special reference to the influence of tobacco habits (1982) Europ J Oral Sci
90: 434-442. https://doi.org/10.1111/j.1600-0722.1982.tb00760.x
- Hedin
C, Pindborg JJ and Axéll T. Disappearance of smoker's melanosis after reducing
smoking (1993) J Oral Pathol Med 22: 228-230. https://doi.org/10.1111/j.1600-0714.1993.tb01061.x
- Halder
RM and Nootheti PK. Ethnic skin disorders overview (2003) J Ameri Acad Dermatol
48: S143-S148. https://doi.org/10.1067/mjd.2003.274
- Silverman
Jr S, Migliorati CA, Lozada-Nur F, Greenspan D and Conant MA. Oral findings in
people with or at high risk for AIDS: a study of 375 homosexual males (1986) J
Ameri Dent Assoc 112: 187-192. https://doi.org/10.14219/jada.archive.1986.0321
- Lozada
F, Silverman Jr S, Migliorati CA, Conant MA and Volberding PA. Oral
manifestations of tumor and opportunistic infections in the Acquired
Immunodeficiency Syndrome (AIDS): findings in 53 homosexual men with Kaposi's
sarcoma (1983) Oral Surg Oral Med Oral Pathol 56: 491-494. https://doi.org/10.1016/0030-4220(83)90095-6
- Hemminki
A, Tomlinson I, Markie D, Järvinen H, Sistonen P, et al. Localization of a
susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative
genomic hybridization and targeted linkage analysis (1997) Nature Genet 15:
87-90. https://doi.org/10.1038/ng0197-87
- Hemminki
A, Markie D, Tomlinson I, Avizienyte E, Roth S, et al. A serine/threonine
kinase gene defective in Peutz-Jeghers syndrome (1998) Nature 391:
184-187. https://doi.org/10.1038/34432
- Meleti
M, Vescovi P, Mooi WJ and van der Waal I. Pigmented lesions of the oral mucosa
and perioral tissues: a flow-chart for the diagnosis and some recommendations
for the management (2008) Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol
105: 606-616. https://doi.org/10.1016/j.tripleo.2007.07.047
- Gaeta
GM, Satriano RA and Baroni A. Oral pigmented lesions (2002) Clinics Dermatol
20: 286-288. https://doi.org/10.1016/s0738-081x(02)00225-0
- Alawi
F. Pigmented lesions of the oral cavity: an update (2013) Dent Clini 57:
699-710. https://doi.org/10.1016/j.cden.2013.07.006
- Kim H.
Generalized oral and cutaneous hyperpigmentation in Addison's disease (1988)
Trop Odonto-Stomatol=Trop Dent J 11: 87-90.
- Nieman
LK and Chanco Turner ML. Addison's disease (2006) Clin Dermatol 24: 276-280.
- Lanza
A, Heulfe I, Perillo L, Dell’Ermo A and Cirillo N. Oral pigmentation as a sign
of Addison’s disease: a brief reappraisal (2009) Open Dermatol J 3: 3-6. https://doi.org/10.2174/1874372200903010003
- Addison
T. On the constitutional and local effects of disease of the supura-renal
capsules (1855) Wellcome Collection, United Kingdom.
- Simpson
SL. Addison's disease (1950) Brit Med J 2: 1164.
- Ten S,
New M and Maclaren N. Addison’s disease (2001) J Clini Endocrinol Metabol 86:
2909-2922. https://doi.org/10.1210/jcem.86.7.7636
- Burk
CJ, Ciocca G, Heath CR, Duarte A, Dohil M, et al. Addison's disease, diffuse
skin, and mucosal hyperpigmenation with subtle "flu-like" symptoms-a
report of two cases (2008) Pediatr Dermatol 25: 215-218. https://doi.org/10.1111/j.1525-1470.2008.00637.x
- Dunlop
D. Eight-six Cases of Addison's Disease (1963) Brit Med J 2: 887.
- Shah
SS, Oh CH, Coffin SE and Yan AC. Addisonian pigmentation of the oral mucosa
(2005) Cutis, United States 76: 97.
- Haydar
NA, Marc JRS, Reddy WJ, Laidlaw JC and Thorn GW. Adrenocortical insufficiency
with normal basal levels of urinary 17-hydroxycorticoids: diagnostic implications
(1958) J Clini Endocrinol Metabol 18: 121-133. https://doi.org/10.1210/jcem-18-2-121
- Najjar
SS and Jarrah A. Pigmentation in Addison's disease; A Case in which
pigmentation was the only sign (1964) Am J Dis Child 107: 198-201. https://doi.org/10.1001/archpedi.1964.02080060200018
- Smith
H. Compensated adrenocortical failure (1963) Lancet 281: 1077-1078. https://doi.org/10.1016/s0140-6736(63)92114-7
- Strakosch C and Gordon R. Early diagnosis of Addison's disease; pigmentation as sole symptom (1978) Austra New Zeal J Med 8: 189-190.https://doi.org/10.1111/j.1445-5994.1978.tb04510.x
Corresponding author
Firoozeh Samim, Assistant
Professor, Faculty of Dentistry, McGill University, 2001 McGill College, RM
502, Montreal, Quebec H3A 1G1, Canada, E-mail: firoozeh.samim@mcgill.ca
Citation
Botros J and Samim F. A patient with a
sudden onset of oral pigmentation (2020) Dental Res Manag 4: 60-65.
Keywords
Oral medicine, Diagnosis, Oral, Pigmentation,
Addison disease


 PDF
PDF