Case Report :
Vilma AlejandraUmanzor Bonilla, Claudette Arambú, Hugo Romero and Juan Jose Guifarro Plasma cell gingivitis is a benign lesion of
unknown etiology characterized by massive infiltration of plasma cells into the
connective tissue of the gingiva. Clinically it presents as a gingival
enlargement with erythema and some areas with the presence of desquamation, it
is usually asymptomatic, but on some occasions the patient may present pain and
gingival bleeding. Diagnosis requires clinical-pathological correlation. Based
on the foregoing, we present a case report of a 25-year-old female patient
diagnosed with plasma cell gingivitis with idiopathic etiology based on the
clinical and histopathological study. Plasma cell
gingivitis is also known as atypical gingivostomatitis,
idiopathic gingivitis and allergic
gingivitis. It is a rare benign condition of the gingiva of
unknown etiopathogenesis, characterized by the proliferation of plasma cells in
the connective tissue. Identified for the first time as a mucosal
hypersensitivity reaction associated with secondary
cheilitis in the 1940s and 1950s, it had its peak between 1966 and 1971, with more
than 50 cases reported [1-5]. Its relationship
with an unknown allergen present in different chewing gums flavored with
cinnamon and mint was established. The pathology is mentioned as a transitory
syndrome that is attributed to purification in the formula of different
products such as chewing gums, sweets and toothpastes that until now appear as
the main etiological suspects. Some reports also suggest plaque as an
allergenic factor [6-8]. Clinically it presents as an erythematous enlargement
that affects marginal and adherent gingiva, generally accompanied by epithelial
desquamation, and the patient generally complains of
pain, tenderness, and bleeding when brushing. The diagnosis
requires a histopathological study characterized by epithelial hyperplasia with
an underlying stroma with the presence of a dense chronic inflammatory
infiltrate in which plasma cells of normal morphology predominate [9]. The
clinical pathologic correlation is essential to differentiate from clinically
similar lesions such as pemphigus, membrane pemphigoid, allergic or lichenoid
reaction, leukemia. Histologically, it can mimic multiple myeloma and solitary
plasmacytoma [10].The objective of this report is to present the clinical case
of idiopathic plasma cell gingivitis and its clinical characteristics,
histopathological study with immunohistochemistry. A 25-year-old
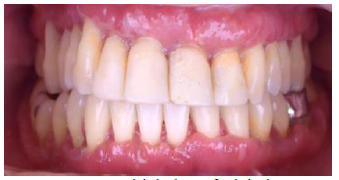
female patient who attended the private practice for the main reason for the
consultation who presented desquamative erythematous gingival hyperplasia with
generalized bone loss with an evolution time of 8 years refers to undergoing
multiple nonsurgical and
surgical periodontal treatments without
responding to this therapy (Figure 1,
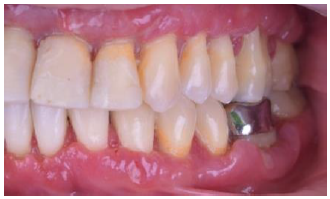
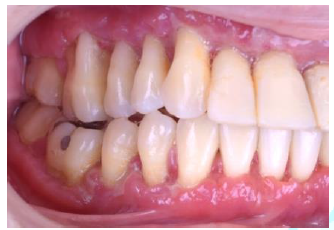
Figure 2 and Figure 3). Figure1:Initial photo facial view. Figure2:Initial photo right side view. Figure3:Initial photo left side view. The patient has
no relevant medical history and complementary laboratory tests showed normal
levels, so leukemia or other hematological alterations are ruled out. The extra
oral clinical examination showed no changes in skin color, facial symmetry and
no lymphadenopathy were palpated, the intraoral clinical examination showed
generalized inflammation and a complete periodontal probing was performed in
which there is an interproximal clinical insertion loss. Detectable in 2 or
more teeth and there is clinical attachment loss >3 mm or with a pocket >3
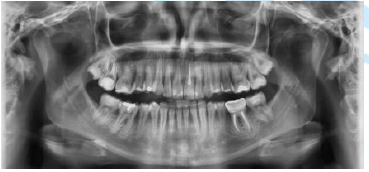
mm in 2 or more teeth. The periodontal diagnosis is stage III periodontitis
because it presents a radiographic interproximal insertion loss >5 mm, a
generalized horizontal bone loss can be observed that extends to the middle of
the roots >30% of the teeth, there is furcation of the teeth 16, 14, 24, 26,
36 and 46 (Figure 4). Figure4:Panoramic radiography. There is the
possibility of losing teeth, the chewing function is preserved and the treatment of
periodontitis does not require complex rehabilitation
of the function. Regarding the degree of progression, it is classified as grade
C, there is indirect evidence of bone destruction is not consistent with the
amounts of plaque and calculus, some destruction patterns suggest periods of
rapid progression, and it has not responded to periodontal therapeutic
controls. No other lesions were observed in the oral cavity. The presence of a
negative Nikolsky sign and the absence of cutaneous lesions ruled out mucocutaneous
diseases. Due to the
clinical aspect, time of evolution, not responding to periodontal therapies and
that a definitive etiological factor was not known, an incisional biopsy was
performed, under a local anesthesia was given with 4% articaine with 1: 100.00
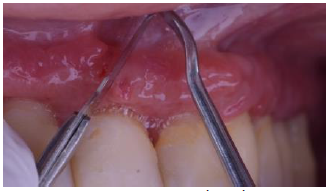
epinephrine. The biopsy was chosen between pieces 21 and 22, taking a
representative lesion with tissue from the lesion and healthy tissue at the apical
edge of these (Figure 5). Exodontia
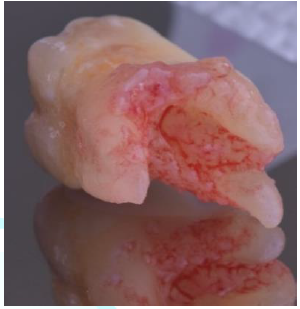
was carried out on tooth 16 that showed tooth mobility; it was observed at the
time of extraction that the tooth was surrounded by granulation tissue (Figure 6). Both were subjected to
histopathological study. Figure6:Exodontia of tooth 16 shows granulation tissue at the apical level. The
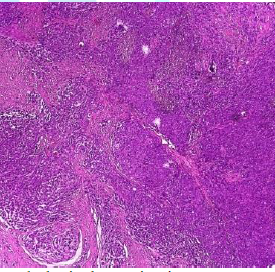
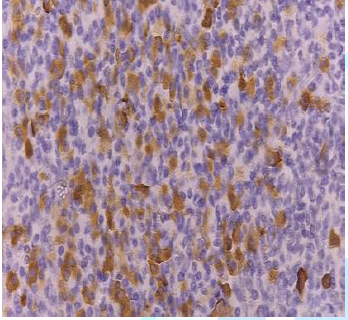
histopathological study in hematoxylin and eosin showed the presence of
hyperplastic parakeratinized stratified squamous epithelium on a stroma that
presents a dense inflammatory infiltrate with a predominance of plasma cells (Figure 7a and Figure 7b). The
histopathological result confirms the diagnosis of plasma cell gingivitis.
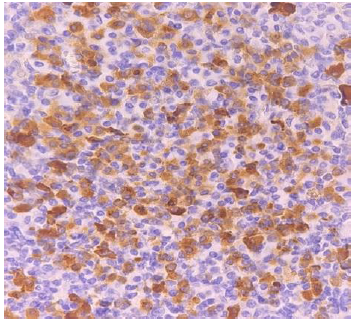
Immunohistochemical markers for kappa and lambda light chains were performed to
confirm the presence of plasma cells and reaffirm the diagnosis (Figure 8a and Figure 8b). Figure7a:Histopathological examination at 10x magnification shows subepithelial inflammation. Figure8a:Immunohistochemical marker expresses positivity for the kappa light chain ƙ. Figure8b: Immunohistochemical marker expresses for ƛ lambda light chain.. Fifteen days
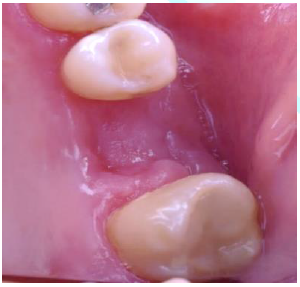
postoperatively after taking the biopsy, the patient attended a follow-up
appointment for assessment, showing in the extraction area of tooth 16 a
gingiva with a decrease in size and volume, as well as in the intensity of
staining (Figure 9), the lesions In
the anterior region where the incisional biopsy was taken which showed no signs
of any recurrence of the enlargement. Figure 9: Postoperative follow-up. The management of
plasma cell gingivitis is directed to the symptoms that the patient have,
corticosteroid treatment is the most frequently used. Plasma cell gingivitis is
a rare pathology that can be similar to multiple benign and neoplastic
conditions, so a correct anamnesis and multidisciplinary investigation are
necessary for its diagnosis. Plasma cell
gingivitis is a rare benign condition of unknown etiology [11]. It is described
as a benign, painful mucosite of plasma cells in the gingiva [1]. Clinically,
it presents as an edematous and erythematous
gingival enlargement in the maxillary and mandibular
segments [12]. The etiology of this pathology is unknown, due to the intense
presence of inflammatory cells it is believed that its origin is due to an
allergic reaction as possible allergens have been associated with gum
flavorings, toothpastes and menthol mouthwashes [6]. The differential diagnosis
of these lesions is important due to their similarity to other pathologies;
other lesions such as mucocutaneous vesicles in the absence of Nikolsky's sign
and other malignant lesions were ruled out by histopathological,
immunohistochemical and complementary hematological examinations [13,14]. Histopathological
results showed a connective tissue with
a dense inflammatory infiltrate, mostly plasma cells, confirming the diagnosis
of plasma cell gingivitis [15]. Confirmation of plasma cell gingivitis requires
immunohistochemistry showing polyclonal expression of the Kappa and Lambda
chains which are free light chains of immunoglobulins that are considered
markers of plasma cell activation. The results of the immunohistochemistry
determined the polyclonal expression of the kappa and lambda light chains in a
ratio of 2:1 suggestive of an inflammatory etiology [16]. Monoclonal
expressions of plasma cells are observed in neoplasms such as multiple myeloma
and extramedullary plasmacytoma in a ratio of 10:1 [17]. Three types of
plasma cell gingivitis have been described: caused by an allergen (flavored
gums, toothpaste, and mint-flavored mouthwashes), neoplastic and of unknown
etiology [18-20]. The management of patients with plasma cell gingivitis is
based on their symptoms, possible allergens and plaque control, which, as in
our case, led to periodontitis and should be eliminated with the intention of
observing possible causes. Plasma cell
gingivitis is an unknown pathology and is occasionally reported in the
literature, it is important as dental personnel to recognize this pathology in
which its diagnosis is based on the clinical-pathological correlation, as well
as interdisciplinary management between the different diagnostic specialties
for the correct treatment plan. Claudette Arambú, Oral Pathology
and Diagnostic Means, Universidad Católica de Honduras Tegucigalpa, Honduras,
E-mail: patologíaoralhn@gmail.com
Bonilla VAU, Arambu C, Romero H
and Guifarro JJ. Plasma cell gingivitis as a predisposing factor for
plaque-induced periodontitis: A case report (2021) Dental Res Manag 4: 71-74. Plasma cell gingivitis, Periodontal
ligament, Periodontitis, Oral pathologyPlasma Cell Gingivitis as a Predisposing Factor for Plaque-Induced Periodontitis: A Case Report
Abstract
Full-Text
Introduction
Case Report

Outcome
Discussion
References
Corresponding
author
Citation
Keywords