Research Article :
Ahmed S Ashour and
Dina A khairy Dysfunctions of Muscles of Mastication (MM) are
commonly associated with facial pain, and it is a common medical condition in
women's reproductive health. Hypothetically, sex hormones could be considered
an underlying cause for this dysfunction, but few studies were done to explore
sex hormones receptors in MM. The aim of the present study is to explore the
effect of both age and sex on the expression of estrogen and androgen receptors
in muscles of mastication. Eighty rats were randomly assigned into four groups.
Group-12F, group-12M, group-24F and group-24M. After rats were sacrificed, MM
were removed for histological and immunohistochemical examinations. Regardless
age and sex, there was a weak expression of estrogen receptors (α,β) in all
muscles. In group-24M, expression of androgen receptors in MM was significantly
higher than that of other groups. In conclusion, the present study sheds the
light on the age-related increased expression of androgen receptors in male
albino wistar rats which could protect against temporomandibular muscles
dysfunctions. Further studies are needed to evaluate this hypothesis for
further clinical applications. Muscles
of Mastication (MM) dysfunctions is commonly associated with myofascial
pain and it is more common in women (aged fifteen - fifty years) [1]. It was
suggested that it is caused by gonado-corticoids and gonadal steroid fluctuations
during menstrual cycle [2]. Other reports mentioned that women receiving
contraceptive pills may suffer from MM dysfunctions which were attributed to
the presence of estrogen receptors in either MM or supplying nerve fibers
modulating the encoding of noxious stimuli, while many reports attributed the
MM dysfunction to the presence of androgen receptors MM [3-5]. It was reported
that testosterone modulates the trigeminal nerve fibers development together with
digastric muscle reflexes [6]. Therefore, during the menstrual cycle, the
fluctuation of sex
hormones levels may cause MM dysfunction and injury [7]. It is also
noteworthy that age is considered another parameter affecting MM [8]. Testosterone
was used as a protective agent to MM in men aged sixty years in various
clinical studies [9]. It was mentioned that sex hormones play a role in MM
dysfunction [10]. But, for the best of our knowledge, number of sex hormones
receptors in addition to the aging effect on such receptors are scanty.
Therefore, this study was designed to shed the light on sex hormones
(especially testosterone and estrogen) receptors expression in MM of both
genders and on the effect of age on such receptors. Chemicals All chemicals and
reagents, unless otherwise specified, were purchased from Sigma-Aldrich
Chemical Co. (Missouri, USA). Animals Eighty albino
wistar rats were used, Average age 12-24 months. Animals were individually
housed. Free access to food and water was allowed. 12 light/dark cycles was
kept. By the help of air conditions, the temperature was kept 25oC
(in accordance to national and institutional guidelines). Experimental design Rats were equally
divided into four groups (n=20). Group-12F composed of 12-months female
rats. Group-12M composed of 12-months male rats. Group-24F composed of
24-months female rats. Group-24M composed of 24-months male rats. As per
protocol described by Marcondes et al. [11], estrous cycle phases were
determined to by analyzing vaginal secretions daily for fourteen days to ensure
the presence of proestrus phase (only female rats with proestrus phases were
included in the current study). Rats were allowed to acclimatize for two weeks
in the animal house before scarification (by intraperitoneally injection of
xylazine (single dose, 15 mg/kg) and ketamine (single dose, 30 mg/kg) (Bio-Veta®,
USA) and MM dissection (masseter, temporalis, pterygoid, digastric muscles)
[12]. Histopathological examination Hematoxylin
and eosin staining was done in accordance to Feldman and Wolfe [13]
protocol. Briefly, fresh muscles tissue was cut into 1cm3
immediately after extraction from the rats. It was placed in fixative 4%
formalin and left for 48 hours then placed in tissue processing cassettes. By
help of ascending grades of alcohol, tissue is dehydrated to remove water and
formalin traces from tissue then immersed in xylene to remove alcohol and
facilitate paraffin wax infiltration into the muscles tissue. Cassettes were
placed on warm plates then tissue was removed and immersed in paraffin blocks.
After paraffin solidification, the blocks were cut into 5 μm thick sections by
using manually operated rotary microtome CUT 4050 (4050F, R) (Microtec
Laborgeräte GMBH, Germany). Tissue sections were placed on glass microscope
slides, rehydrated, stained with hematoxylin (stains nuclei in blue) for 10
minutes and eosin (stains cytoplasm in red) for 10 seconds. The stained tissue
sections were dehydrated again by ascending grades of alcohol for 10 minutes
than covered by coverslip. Histopathological examinations were performed by two
expert histopathologists blinded to our study (ten overlapped fields per
section) then the images were analyzed by Image J. 1.24 v. software (Volumetry®,
USA) Immunohistochemistry examinations Immunohistochemistry
was done in accordance to Ward and Rehg [14] protocol. Briefly, paraffin
embedded tissue sections were sliced (5 μm thick) and mounted to charged
slides. Sections were deparaffinized and rehydrated by descending grades of
alcohol. Endogenous peroxidase activity was quenched by placing the tissue
sections in 3% hydrogen peroxide for 10 minutes. 200 μl of diluted 1ry antibody
[anti-estrogen receptor-α (ER-α) antibody, dilution 1:1000; anti-estrogen
receptor-β (ER-β) antibody, dilution 1:1000; Anti-Androgen Receptor (AR)
antibody (dilution 1:500), (ABCAM®, USA)] were mounted to the
tissue. Slides were incubated overnight at 4oC in a humidified
chamber. In next morning, slides were washed by wash buffer for 3 minutes then
covered with 2 drops of Signal Stain Boost Detection Reagent followed by
incubation at room temperature in humidified chamber for 30 minutes. 200 μl of
Signal Stain® DAB (Biocompare, USA) were applied to each section.
After staining, slides were immersed in distilled water then counterstained
with haematoxylin to stain nuclei in blue for better visualization. Coverslips
were applied, examinations were performed by to expert histopathologists
blinded to our study scoring was done as follows: 0=negative,
1=weak, 2=mild, 3=moderate, 4=strong reaction. Ten fields per section were
analyzed by Image J 1.24 v. software. Statistical analysis Statistical
Package for Social Sciences (SPSS) software, 20 V. (SPSS Inc., USA) was
used for data analysis. The statistical significance of differences between
groups was validated using One-Way Analysis of Variance (ANOVA). Post hoc
Tukey-Kramer test was used for groups’ comparison. Data were expressed in mean
± Standard
Deviation (SD) and probability value was considered significant if
<0.05. Effect of age and sex on masticatory
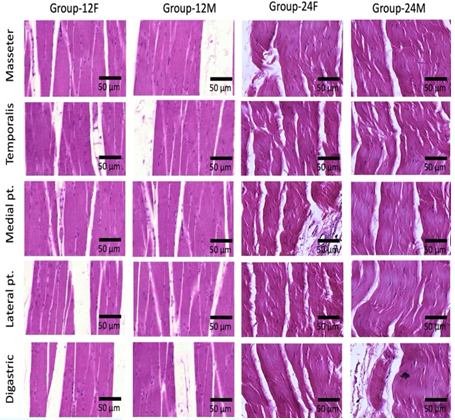
muscles ultrastructure Light microscopy
examination of rat’ MM tissue sections stained with H and E showed normal
histological architecture of different muscles of mastication in both group-12F
and group-12M. Myofilaments appeared with neat arrangement with peripherally
located nuclei. Group-24F and group-24M showed areas of muscle atrophy appeared
in the form of wavy arranged myofilaments, hyper eosinophilic sarcoplasm,
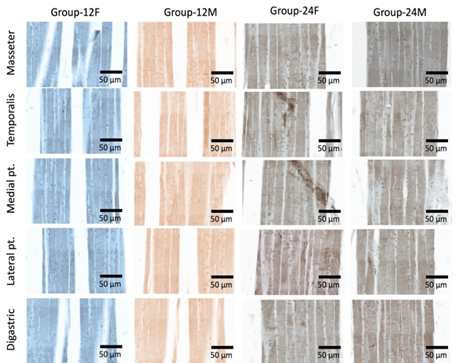
crowded nuclei with inflammatory cells infiltrations (Figure 1). Effect of age and sex on ER-α and ER- β
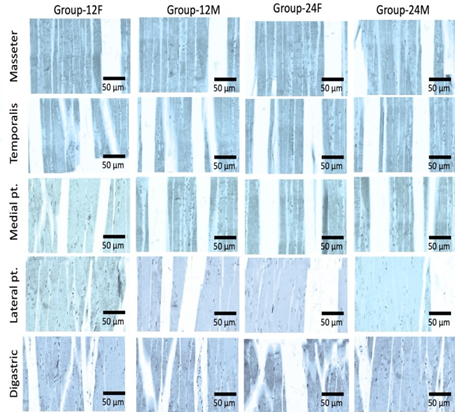
receptors Light microscopy
examination of rat’ MM tissue sections stained with anti-ER-α and ER-β
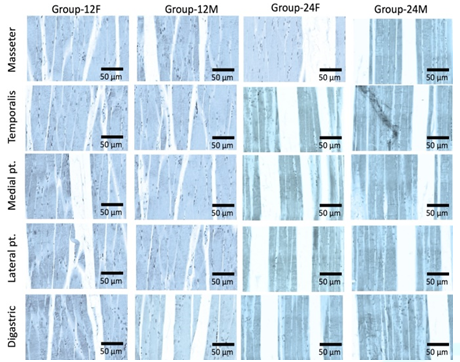
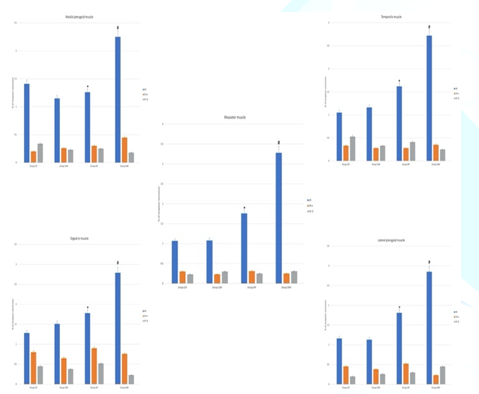
antibodies showed low expression in different groups and muscles (Figures 2-4). Effect of age and sex on AR receptors Light microscopy
examination of rat’ MM tissue sections stained with anti-AR antibodies showed
significant AR expression group-24M (especially in masseter muscle) if compared
to other groups. In addition, group-24M showed that AR expression in the
masseter muscle was significantly higher than if compared to digastric muscle,
there were non-significant differences between other muscles of mastication (Figures 4-5). Wang et al. [7]
reported that dysfunctions of mastication muscles are presented with muscle
pain and difficulties in mastication, Widmer et al. [5] attributed the
age-related difficulties in speech and swallowing to MM dysfunctions which
becomes more frequent in women (aged fifteen - fifty years) [15]. Current study
results showed that androgen receptors located in MM may guard against
age-related dysfunctions due to activation of anabolic pathway which results
from its expression which could explain the vulnerability of females to these
muscle related dysfunctions which comes in agreement with Iacovides et al. [15]
who attributed the abundant presence of type II muscle fibers (fast-twitch)
(essential for mastication movement) in masseter muscle to the activated
expression of androgen receptor [12,13,16]. Shi et al. [17]
reported that masseter muscle atrophy may cause Temporomandibular
Joint (TMJ) movement disorder which depends primarily on the latter fibers.
Baig et al. [18] reported that sustained androgen
receptors expression with age helps in the maintenance of capillary network
density in muscles. Iacovides et al. [15] reported the role of androgen
receptors in increasing the mass of muscles (by enhancing type I and type II
fibers growth) which results in muscular trophism and structural maintenance
and linked between the short contraction intervals in the male rabbits’
masseter muscle and the activated androgen receptors which sheds the light on
the role played by these receptors in enhancing the physiological properties of
MM as well which delay muscle fatigue resulting in less MM dysfunctions [14]. The present study
showed that androgen receptor expression was highest in the masseter muscle of
group-24M which may compensate to the age-related reduction in circulating
testosterone which comes in agreement with Wilson et al. [19] who reported that
serum testosterone levels drops to 25% of its normal value in old rats aged two
years. Circulating Serum Testosterone (ST) is bound to Sex
Hormone-Binding Globulin (SHBG) [affecting hormonal distribution and hence
its biological activity] the latter functions and levels may be affected in
malnutrition and obesity [20]. English and Widmer [21] reported the age-related
increased activity of 5α-reductase enzyme (5α-R) in the prostatic acinar
epithelium, which facilitate the irreversible conversion of testosterone into
dihydrotestosterone (DHT), the latter has a more potent agonist effect on
androgen receptors. Some medications such as finasteride (proscar®),
may reduce 5α-R activity in addition to androgen receptor affinity to ST. Ekenros et al.
[16] reported the anti-oxidant and anti-inflammatory potential of androgens
which may also hinder age related MM-dysfunctions. ST is also crucial for the
maturation of digastric muscle reflexes through increasing the neuronal density
in the caudal part of trigeminal nerve nucleus extending through brain stem
reflecting the protective role of testosterone against temporomandibular
muscles dysfunction [18]. Many studies were done to explore the effect of
testosterone on temporomandibular joint structural and functional properties
and to identify the reasons of high prevalence of females to temporomandibular
dysfunctions [13]. The androgen receptors in masticatory muscles could be
considered the line of defense. In conclusion,
the present study sheds the light on the age-related increased expression of
androgen receptors in male albino Wistar
rats which could protect against temporomandibular
muscles dysfunctions. Further studies are needed to evaluate this
hypothesis for further clinical applications. Ahmed S Ashour, Department of
Biomedical Dental Sciences, College of Dentistry, Imam Abdulrahman Bin Faisal
University, Kingdom of Saudi Arabia, E-mail: asashour@iau.edu.sa
Ashour AS and Khairy DA. Expression
of androgen receptors in abating age-related temporomandibular muscles
dysfunctions in female albino Wistar rats (2021) Dental Res Manag 5: 8-11. Androgen receptors, Muscles of mastication,
Temporomandibular dysfunction, Aging, EstrogenExpression of Androgen Receptors in Abating Age-Related Temporomandibular Muscles Dysfunctions in Female Albino Wistar Rats
Abstract
Full-Text
Introduction
Materials and Methods
Results
Discussion
References
Corresponding
author
Citation
Keywords