Research Article :
David Jaramillo,
Jose L Ibarrola, Ana Arias, Phillipe Sleiman,
Ali Naji and David E Jaramillo Objective: The objective of this study was to
observe the effect that 3% Sodium Hypochlorite and 17% Ethylenediamine Tetraacetic
Acid (EDTA) with water in between, used sequentially and individually had in
the disinfection of dentin blocks that were contaminated with Enterococcus faecalis. Materials and Methods: Thirty apical and coronal dentin block
samples were divided into five groups (n=5): All samples were inoculated with Enterococcus faecalis Group 1: Samples
were submerged in 3% NaOCl then in 17% EDTA. Group 2: Samples were submerged
first in 17% EDTA and then in 3% NaOCl. Group 3: Samples were immersed in 3%
NaOCl only. Group 4: Samples were submerged in 17% EDTA only. Group 5 (positive
control group): Samples were only submerged in distilled water. All samples
were submerged in distilled water after each irrigation cycle. The irrigation
protocol was repeated in all groups until all dentin samples were exposed to
24ml of the irrigating solutions. CFU units were counted and classified in an
ordinal scale and compared with the linear-by-linear association test. Results: A significant linear trend in the
reduction of CFU was observed when NaOCl and EDTA were combined (independently
of the sequence used) when compared to groups 3 and 4 where the irrigants were
used individually both in coronal (p=9.45 x 10-21), and apical slices (p=2.33 x
10-20). NaOCl was significantly more effective than EDTA in both
coronal and apical slices (p ≥ 0.000001) when used alone. Conclusion: 3% Sodium Hypochlorite was more
effective than 17% EDTA. However, alternating 3% NaOCl with 17% EDTA resulted
in better dentinal disinfection. Clinical Relevance: Establishing an irrigation protocol that is
effective in eradicating bacteria entrenched in dentinal tubules can lead to a
more successful outcome in endodontically treated teeth. The relationship
between pulpal and periradicular pathology and microorganisms has been well
established [1-2]. Endodontic therapy aims to at reduce as much as possible the
bacterial load of contaminated root canal systems. Numerous techniques and
antimicrobial agents have been suggested and utilized to achieve this goal [3].
Sodium
Hypochlorite (NaOCl) as a sole irrigation agent at
first or in combination with other solutions has been the most widely utilized
irrigant in root canal
therapy. Factors such as concentration of the solution,
time of contact with microorganisms, and irrigation volume are important
factors in the effectiveness of irrigating solutions. NaOCl is used in
concentrations ranging from 0.5% to 6%. NaOCl is a potent antimicrobial agent
and has the capacity to dissolve and digest pulpal tissue remnants [4]. Enterococcus faecalis is a
facultative anaerobic gram-positive coccus that is resistant to intracanal
medications and able to invade dentinal tubules [5]. Vieira et al [6] described
dentinal tubule infection as a cause of endodontic treatment failure. Gomes et
al. [7] tested the effect of various concentrations of NaOCl in vitro on Enterococcus faecalis and found it to be
efficient in killing the bacteria in less than 30 seconds while using a 5.25%
NaOCl solution, conversely when using a 0.5% solution of NaOCl it took 30
minutes to eliminate E. faecalis colonies.
To achieve their maximum antimicrobial
efficacy irrigation solutions must come in
direct contact with microorganisms, dentin debris can impede NaOCl from direct
contact with bacteria thus reducing its efficacy [8]. Root canal anatomy can
present an obstacle in preventing thorough chemo-mechanical preparation of the
root canal space [9]. Furthermore, bacteria can become imbedded deep inside
dentinal tubules where they cannot be reached by the antibacterial solutions
[10-11]. Cleaning and shaping procedures have been shown to heighten this
problem by producing debris deposits that contribute to the formation of smear
layer [12]. In addition, dentin itself can have a neutralizing effect on root canal
medicaments. Haapasalo et al [13] showed that the
presence of dentin delayed the effectiveness of 1% NaOCl in eliminating E. faecalis. Also, it has been shown
that NaOCl alone is unable to remove the smear layer [14]. At the same time,
the use of intracanal
chelator solutions such as EDTA have been shown to be
effective in the removal of the inorganic portion of the smear layer thus
making the dentinal tubules more accessible to irrigation solutions and
improving the bacterial effectiveness of disinfecting solutions in deeper
layers of dentin [10,15]. EDTA and NaOCl have been shown to be more effective
in disinfecting and cleaning dentinal tubules when used sequentially than when
used as independent agents. Wang et al [16] demonstrated that alternating the
use of NaOCl and EDTA with water in between is more efficient in keeping
dentinal tubules open during cleaning and shaping procedures that when using
NaOCl or EDTA alone. While EDTA does not possess significant antimicrobial
properties, it can enhance the effectiveness of other antimicrobial agents by
allowing their dissemination into otherwise inaccessible dentinal tubules that
can potentially harbor microorganisms [17]. The aim of this study was to
analyze the effect of different irrigation protocols that included 3% Sodium
Hypochlorite and 17% EDTA, used both sequentially and individually, in the
disinfection of dentin blocks contaminated with E. faecalis. It is also of interest to see if increasing the volume
and contact time with these irrigants would influence dentin disinfection. Sample collection and sample preparation Thirty dentinal block
slices each measuring 3mm x 5 mm were obtained from teeth
(maxillary 1 rooted premolars) collected from the Oral and Maxillofacial
Surgery and Periodontics departments at the University of Texas Health Science
Center at Houston, Texas. Dentin sample sections measuring 3 X 5 mm were
obtained with an Isomet 1000 precision saw (Buehler, Lake Bluff, IL). After the
sectioning procedure was completed each dentin sample was submerged in a
micro-test tube containing 17% EDTA to remove any residual smear layer that
might have been produced during sectioning. All samples were autoclaved at a
temperature of 121°C at 20 PSI for 20 minutes. The specimens were
then placed in 250-ml Erlenmeyer flasks (Nova Tech International, Kingwood, TX)
filled with Brain Heart Infusion (BHI) media and inoculated with E. faecalis, to an optical density of 1
(=20 million bacterial units). The flasks were placed in an incubator (Shel
Lab, Vernon Hills, IL) for a period of three weeks at 37°C. Apical and coronal
samples were obtained from the contaminated dentin blocks and designated and b
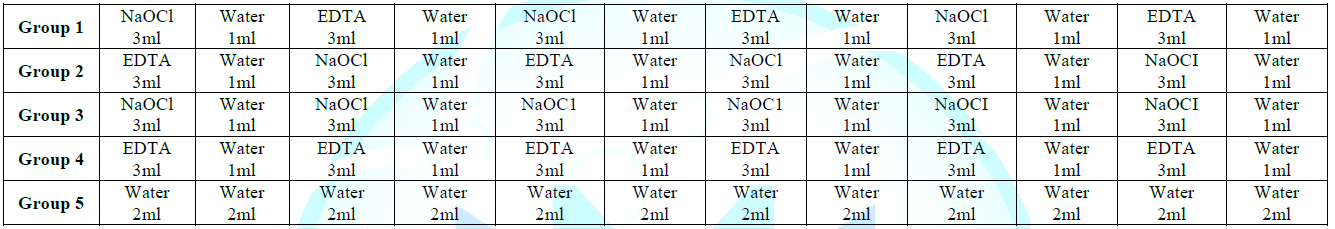
respectively. Experimental groups and controls Depending on the
irrigation protocol used, the samples were randomly divided into four
experimental groups and one positive control group (Table 1) as follow: Group
1:
3% sodium hypochlorite was used followed with immersion in distilled water,
then submerged in 17% EDTA and immersed in distilled water. The process was
repeated until exposure to the irrigants reached 24 ml. Group
2:
17% EDTA was used followed with immersion in distilled water, then submerged in
3% sodium hypochlorite and immersed in distilled water. The process was
repeated until exposure to the irrigants reached 24 ml. Group
3:
Only 3% sodium hypochlorite was used in this group with intermittent exposure
to distilled water. The process was repeated until exposure to the irrigants
reached 24 ml. Group
4:
Only 17% EDTA was used in this group with alternating exposure to distilled
water. The process was repeated until exposure to the irrigants reached 24 ml. Group
5 (positive control group): Only distilled water was used in this
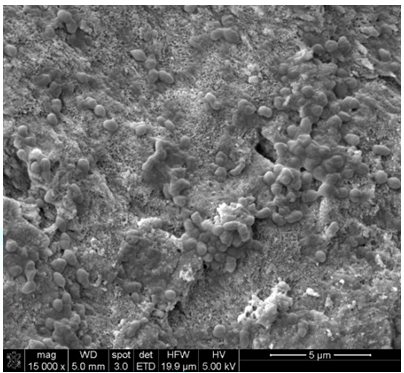
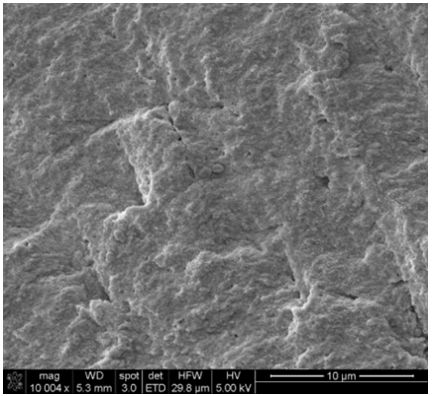
group and was designated as the positive control group (Figure 1 to Figure 5). Figure1:Positive Control. SEM images demonstrated cells growth on top of dentin blocks. Figure3: NaOCl group. NaOCl showed no evidence of debris removal from dentin blocks surface. Figure4: EDTA group. EDTA show some disruption of inorganic debris attached to dentin blocks. Figure5: Water group. Water showed no evidence of debris removal from dentin blocks surface. Table1:Irrigation sequence per group. Dentin block
samples in the experimental groups were individually immersed in 2 ml Eppendorf
tubes (Eppendorf North America, Hauppauge NY) that contained 1ml of the
irrigation solution. The irrigant was replenished every 20 seconds with 1 ml of
fresh solution until the dentin block samples were exposed to 3 ml of the
solution in one minute. The dentin blocks were then submerged in 1 ml of
distilled water for one minute. Samples were then immersed in 1ml of the next
designated irrigating solution and after 20 seconds the irrigant was
replenished with fresh solution until dentin exposure to the irrigant reached 3
ml in one minute. The samples were then immersed in 1 ml of distilled water for
one minute. This sequence was
repeated until all experimental samples were exposed to the irrigating
solutions for a total of 24 ml. One group was designated the positive control
group and was exposed to 2 ml of distilled water which was replenished every
minute until the samples were exposed to 24 ml of water. After each exposure
the samples that were immersed in NaOCl were submerged for five minutes in a 5%
sodium thiosulfate solution to inactivate any NaOCl residues. At the end of the
irrigation cycles all dentin block samples were individually placed in
Eppendorf tubes filled with Phosphate
Buffered Solution (PBS) and agitated with a Vortex Genie
2 mixer. 30 µl of the suspension was collected from each tube and seeded in BHI
agar plates. All samples were incubated for 24 hours and CFU were counted using
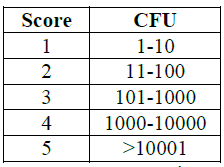
Dahlen et al. [18] methodology. Data was classified as follow: 1=1-10 CFU;
2=11-100 CFU; 3=101-1000 CFU; 4=1001-10000 CFU; 5= + 10000 CFU (Table 2). Statistical Analysis The ordinal
(linear) chi-square test, also known as the linear-by-linear association test,
was used to compare the effectiveness of the different irrigation protocols and
assess any difference in the trend to reduce CFU among groups both for coronal
and apical slices. CFU for all
samples in the positive control group was higher than 100000. Results for each
irrigation regimen for both coronal and apical tooth slices are shown in Table 2.
No significant differences were detected between groups 1 and 2 for apical
(p=1) or coronal slices (p=0.74). However, a significant linear trend in the
reduction of CFU was observed in those groups where NaOCl and EDTA were
combined (independently of the sequence used) when compared to groups 3 and 4
where the irrigants were used individually both coronal (p =9.45 x 10-21),
and apical slices (p=2.33 x 10-20). At the same time, NaOCl as only
irrigant was significantly more effective than EDTA in both coronal and apical
slices (p ≥ 0.000001). This in vitro
study was designed to assess the effectiveness of different irrigation
protocols on dentin block slices contaminated with E. faecalis. A key element on successful endodontic outcomes is the
eradication of microbes from the root canal system. This goal is accomplished
by mechanical instrumentation in conjunction with copious irrigation of the
root canal. However, bacteria can remain in inaccessible parts of the canal
where mechanical instruments and irrigation solutions cannot reach [19]. Once
pulpal necrosis occurs infected dentinal tubules can become an important
reservoir from which canal reinfection can occur [20]. Furthermore, Veira et al
[6] published a case report of a long- term treatment failure that was
attributed to dentinal tubule infection. It has been proposed that bacteria can
penetrate deep into dentinal tubules, making it difficult for irrigating
solutions and medicaments to penetrate and eliminate bacteria from contaminated
dentin [21]. Sedgley [22] observed that E. faecalis could remain viable ex vivo
after treatment and provide a long term-nidus that can induce subsequent endodontic
disease. Therefore, it is important to disinfect not only
the root canal space but
also the surrounding dentin as well. Different irrigation solutions have been
used with reports of varying degrees of success [23]. Factors that affect the
effectiveness of these irrigating solutions are the concentration of the
irrigation solution; volume utilized, delivery method utilized, and contact
time of the irrigation solution with the affected area [3]. This study
compared the effectiveness of irrigation solutions used as single irrigants and
contrasted them to the same irrigation solutions used in combination. The order
in which the irrigation solutions were utilized was exchanged to see if a
certain irrigation protocol was more effective in eliminating bacteria from
dentinal tubules. Distilled water was used as an intermediate irrigant to
reduce the interaction between the different irrigating solutions which could
potentially reduce the irrigant effectiveness or result in the production of
undesirable by-products [24]. Wang et al. [16] showed that alternating the use
of NaOCl and EDTA with distilled water was helpful in preventing accumulation
of smear layer in dentinal tubule orifices than using NaOCl or EDTA alone.
Counting of CFU showed that using a combination of 3% Sodium Hypochlorite with
17% EDTA was more effective in reducing bacterial contamination in dentinal
tubules than using any of these irrigants individually. Bystrom and
Sundqvist [25] evaluated the clinical effectiveness of NaOCl irrigation and
found that the combined use of 5% NaOCl with EDTA was more efficient than using
NaOCl solutions alone. Baumgartner and Mader [26] used scanning electron
microscopy to evaluate the effects that NaOCl and EDTA used individually or in
combination had on the removal of the smear layer and found that NaOCl and EDTA
used in combination was more effective in removing smear layer that when these
chemicals were used individually. However, their study focused on smear layer
removal and not the eradication of microbes entrenched in dentinal tubules. Berutti et al
[27] studied histological sections obtained from dentin samples that had been
artificially infected and found that a combination of 5% NaOCl and 10% EDTA
left residual dentinal infection to a depth of 300µm. The results obtained in
our study contrast with those obtained by Berutti [27]. In this study residual
microorganisms were not detected when 17% EDTA and 3% NaOCl were used in
combination and alternating each cycle with distilled water. This difference
may be explained by the higher irrigation volume and the constant refreshing of
the irrigating solutions. Azim et al. [28] studied the effects of 4 irrigation
protocols in eliminating bacteria from root canals especially in dentinal
tubules, confocal analysis showed that to achieve better disinfection in the
deeper dentin layers a device such as PIPS was needed [28]. Other observations
from this study were that Sodium Hypochlorite at a 3% concentration showed a
reduction in the formation of CFU but not to the degree of alternating NaOCl
with EDTA. Also, using a 17% solution of EDTA as a sole irrigant resulted in a
negligible reduction in the formation of CFU. The positive control group
consisted of irrigation solely with distilled water and had no effect
whatsoever in the formation of CFU. Interestingly
alternating the order in which NaOCl and EDTA did not influence the
effectiveness of the irrigating solutions in the disinfection of dentinal
tubules. This could be explained that by replacing 1 ml of the experimental
irrigating solutions every 20 seconds until exposure to 3ml of solution per
minute was reached allowed the dentin samples to be in continuous contact with
fresh irrigation solution. In addition, each cycle was repeated until each
protocol received 24 ml of total exposure to the irrigating solutions thereby a
higher irrigation volume was achieved while maintaining the freshness of the
solutions utilized. In this study
dentin block samples were obtained from both the apical (a) and coronal (b)
regions of the root to see if the difference in density and size of the
dentinal tubules in the respective regions would have an effect in the capacity
of the irrigating solutions to eradicate microorganisms harbored within the
dentinal tubule lumen, even though apical dentin has smaller and more irregular
dentinal tubules there was no difference in CFU reduction between apical dentin
samples (a) and coronal dentin samples (b) in any of the groups tested [29]. The results of
this study show that constant refreshing of NaOCl and EDTA irrigants, with
water in between can be an effective way of achieving dentin disinfection at
all levels of radicular dentin. Further research should study if the same
results can be achieved with conventional irrigation through the root canal
system particularly after shaping and cleaning procedures. In addition, it has
been shown that prolonged exposure to EDTA can cause excessive removal of peritubular and intratubular
dentin and it would be of interest to see if constant
refreshing of EDTA solutions would have any deleterious effects on the
structural integrity of these tissues [30]. It has been shown that agitating
irrigating solutions can improve the effectiveness of irrigating solution in
eradicating bacteria from the dentinal tubules a study designed to see if
constant replenishing of the irrigating solutions in combination with agitating
aids would increase the effectiveness of these devices would be of interest
since this study showed that repeated replenishing of irrigating solutions will
increase their effectiveness [24]. Also, when using NaOCl, distilled water and
EDTA the order in which these solutions did not affect the results however
altering the irrigation sequence in chemo-mechanically prepared canals may show
different results. A combination of
3% NaOCl and 17% EDTA solutions were more effective in reducing colony forming
units than immersion in individual solutions of either 3% sodium Hypochlorite
or 17% EDTA. The order in which these solutions were used did not have any
effect on the results, rather it was more important to use these solutions in a
dual irrigation protocol. No difference was observed between the samples of
apical or coronal dentin despite the differences of diameter and number of
dentinal tubules at both sites. Conflict of interest Author 1 declares
that he has no conflict of interest. Author 2 declares that he has no conflict
of interest. Author 3 declares that he has no conflict of interest. Author 4
declares that he has no conflict of interest. Author 5 declares that he has no
conflict of interest. Author 6 declares that he has no conflict of interest. Funding The work was supported
by a seed grant (0012625) given to Dr. David E. Jaramillo from the Department
of Endodontics, University of Texas Health Science Center at Houston, USA. Ethic approval This article does
not contain any studies with human participants or animals performed by any of
the authors. All procedures performed in studies involving human participants
were in accordance with the ethical standards of the institutional and/or
national research committee and with the 1964 Helsinki declaration and its
later amendments or comparable ethical standards. The institutional
Review Board (HSC-DB-18-0008) of the University of Texas Health Science Center
at Houston has approved this research. David E Jaramillo, University
of Texas, Health Science Center, 7500 Cambridge St, Houston TX, USA 77054, E-mail:
David.E.Jaramillo@uth.tmc.edu
Jaramillo D, Ibarrola JL, Arias
A, Sleiman P, Naji A, et al. Dentin disinfection efficacy using four different
irrigation protocols (2021) Dental Res Manag 5: 33-37. Endodontics, Root canal irrigation, NaOCl, EDTADentin Disinfection Efficacy Using Four Different Irrigation Protocols
Abstract
Full-Text
Introduction
Materials and Methods



Results
Discussion
Conclusion
Compliance with Ethical Standards
References
Corresponding
author
Citation
Keywords