Diabetic vasculopathy,
including macro and micro vascular disorders, is the leading cause of morbidity
and mortality in patients with type 1 (T1) and type 2 (T2) diabetes mellitus (DM)
[1]. A lot
of researches pointed
out that endothelial
dysfunction, characterized by
an imbalance between
Endothelium-Derived Relaxing Factors
(EDRFs) and endothelium-derived contracting
factors (EDCFs) play
a central role
on the development
and progression of diabetic
vasculopathy [2-5].
Endothelial dysfunction
and inflammation, as
indicated by abnormal
flow-dependent
vasodilatation and by
increased circulating levels
of adhesion molecules
(ICAM-1 and E-selectin) are known to occur in T2DM and seems to be an
important predictor in systemic atherogenesis [6]. Both hyperglycemia
and insulin administration increasing
circulating levels of endothelin-1
(ET-1), an endothelial cell (EC)-derived potent vasoconstrictor peptide with
mitogenic, pro-oxidative and pro-inflammatory properties that have shown to be extremely
relevant to the pathophysiology of diabetic vasculopathy [7-10].Circulating and
local levels of
ET-1 are increased
in diabetic animal
models and diabetic patients [1,11,12]. Considering the
global epidemic of diabetes,
it seems to be critical to update our understanding of
the pathogenesis of
diabetes and related
vascular complications in
order to clearly
understand if an endothelial protector
drug, able to
modulate endothelial adhesion
molecules and ET-1
could represent a
novel treatment options
for prevention and delaying the progression of diabetic complications
[6].
The mechanism regulating endothelial cells and vascular smooth muscle cells function to become an important therapeutic targets in diabetic vascular complications and especially, the modulation of the vasoconstrictor, mitogenic, pro-oxidative and pro-inflammatory properties of ET-1 is undoubtedly important in diabetic complications. As everybody knows the small vessels (microcirculation comprises arterioles, capillaries, venules and lymphatics, all <100 mm in diameter) are crucial for maintaining tissue metabolism and structural and functional changes in the microcirculation are present in diabetes mellitus irrespective of the organ studied (retina, kidney, CNS and skin) [6]. The pathophysiology of diabetic microangiopathy is complex because it involves not only metabolic but also genetic factors [6]. For example has been shown that subjects with diabetes heredity have impaired microvascular responses to both endothelium and nonendothelium-dependent stimuli in the skin microcirculation in spite of normal body dimension, normal glucose tolerance and normal insulin sensitivity [13-15]. Early on in the course of the disease, microvascular perfusion
occurs in the limbs, but most of the blood flow under normal thermal conditions passes through arteriovenous shunts, bypassing the nutritive capillary bed and leading the so-called capillary ischemia [16,17]. Endothelial dysfunction, characterized by an imbalance between endothelium-derived vasodilatator and vasoconstrictor substances, plays an important role in the pathogenesis of vascular complications in diabetes, including microangiopathy. Almost two different steps seem to be involved in the microcirculation imbalance: leukocyte recruitment cascade and Endothelin-1 overexpression [16,18,19].
The recruitment of leukocytes from circulating blood into tissues is crucial for the inflammatory response: during this process a number of well-studied adhesion molecules on the endothelium sequentially interact with their ligands expressed on the cell surface of leukocytes. The interaction between adhesion molecules and ligands occurs in a cascade-like fashion, driving leukocytes from the circulation to the extravascular space, that is, through the steps of leukocyte rolling, firm adhesion and transmigration (Figure 1) [20]. The selectin family of adhesion molecules mediates the capture and rolling steps of leukocytes along the endothelial cells. The selectin consists of three members of C-type lectins (P, E and L-selectin).
After the selectins have initiated leukocyte rolling along the surface of endothelium, a different set of adhesion molecules comes into play to reduce the leukocyte rolling velocity and allow to leukocyte to firmly adhere to the endothelial surface. This firm adhesion step is largely mediated by molecules of immunoglobulin superfamily such as intercellular adhesion molecule (ICAM – 1) and vascular cell adhesion molecule (VCAM-1) expressed by endothelial cells and by those expressed constitutively by leukocyte or by many other types of cells. Upon achievement of stable adhesion to the endothelial surface, the leukocyte extravasate between endothelial cells along the intercellular junctions. PECAM-1 (Platelet Endothelial Cell Adhesion Molecule) and VAP (Vascular Adhesion Protein) mediated leukocytes transmigration [20]. Various lines of evidence indicate that the shedding of selectins is enhanced on the endothelium during the progression of diabetes and that the soluble form of selectin proteins has the potential to be a clinically useful biomarker of the severity of Diabetic Rethinopathy: E-Selectin, in particular, may also serve as a proangiogenic factor [20].
Once that the leukocytes have transmigrated from endothelial junctions a hyperproduction of ET-1 (Endothelin 1) have been released by the endotheliam. ET-1 is one of the most potent vasoconstrictor described and has been suggested to be involved in the development of cardiovascular disease. It possess pro-inflammatory and profibrotic effects [6]. Enhanced of endogenous ET-1 has been demonstrated in hypertension, coronary artery disease and heart failure [6]. In diabetic microangiopathy one important feature of endothelial dysfunction is an increased in production and biological activity of the vasoactive and proinflammatory peptide ET-1. Elevated levels of ET-1 are found in patients with type 2 diabetes. Furthermore ET-1 may contribute to the development of endothelial dysfunction, and consequently insulin resistance, by increasing the production of Reactive Oxigen species, mainly superoxide anion, in the vasculature [6].
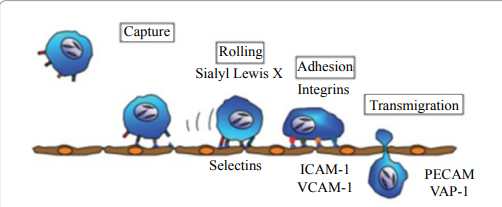
Figure 1: Leucocyte recruitment to the vessel wall
Taking into account the role of endothelial adhesion molecules (specifically E-Selectin) and ET-1 in the pathogenesis of diabetic microangiopathy and that mostly of the diabetic complications such as retinopathy, nephropathy and neuropathy have their basis in disturbed microvascular function, we hypnotized that added to standard therapy an endothelial protector drug, able to counteract hyperespression of endothelial adhesion molecules and ET-1 could be a new promising idea to postpone diabetic microvascular complication.
Recent published and not published studies shown that an endothelial protecting drug, such as aminapthone (2-hydroxy-3-methyl-1,4-napthohydroquinone-2-p-aminobenzoate), a synthetic molecules derived from four aminobenzoic acid which is currently employed for capillary disorders could be useful in reverse microalbuminuria and in control nailfold periungueal videocapillaroscopy and retinal impairment (OCT and fluoroangiography) in diabetic patients [21,22].
Considering that recently aminapthone shown a very interesting direct pharmacodinamic profile on endothelial cells (improvement of E-selectin and ET-1 hyperespression) and that other drugs like avosentan (a new potent, non peptidergic and selective Et-a receptor antagonist) demonstrated to decrease proteinuria after 3 – 6 months of treatment, it seems encouraging to study if this new endothelial therapeutic approach could be useful for diabetic patients when added to standard therapy [23-29].
Since the typical approach with anti- ET-a selective antagonist avosertan, atrasentan and sitaxsertan seems to be encouraging in term of efficacy (proteinuria control in diabetic patients) but not in term of safety (increased of morbidity and mortality associated with anti-ET-a selective antagonists induced fluid retention) an old and safe endothelial protector approach with aminapthone could represents a new/old way to postpone diabetic microangiopathy complications [27-29].
References
1. Matsumoto T, Noguchi E, Kobayashi T, Kamata
K. Mechanisms underlying the
chronic pioglitazone treatment-induced improvement
in the impaired
endothelium-dependent relaxation seen in aortas from diabetic rats
(2007) Free Radic Biol Med 42: 993-1007.
2. Forbes JM, Cooper ME. Mechanisms of diabetic
complications. Physiol Rev 93: 137-188.
3. Mather
KJ The vascular
endothelium in diabetes--a
therapeutic target? (2013) Rev
Endocr Metab Disord 14: 87-99.
4. Muniyappa
R, Sowers JR.
Role of insulin
resistance in endothelial dysfunction (2013) Rev Endocr
Metab Disord 14: 5-12.
5. Sowers JR. Diabetes mellitus and vascular
disease (2013) Hypertension 61: 943-947.
6. Kalani M.
The importance of endothelin-1 for microvascular dysfunction in diabetes
(2008) Vasc Health Risk Manag 4: 1061-1068.
7. Callera GE, Tostes
RC, Yogi A, Montezano AC, Touyz RM. Endothelin-1-induced oxidative
stress in DOCA-salt
hypertension involves NADPH-oxidase-indipendent mechanisms (2006)
Clin Sci 110: 243-253.
8. Tostes RC, Muscará MN. Endothelin receptor
antagonists: another potential alternative for cardiovascular diseases (2005)
Curr Drug Targets Cardiovasc Haematol Disord 5: 287-301.
9. Kohan DE, Rossi NF, Inscho EW, Pollock DM.
Regulation of blood pressure and salt homeostasis by endothelin (2011) Physiol
Rev 91: 1-77.
10. Ferri C, Pittoni V, Piccoli A, Laurenti O,
Cassone MR, et al. Insulin stimulates endothelin-1 secretion
from human endothelial
cells and modulates
its circulating levels in vivo (1995) J Clin Endocrinol Metab 80:
829-835.
11. Kanie
N, Matsumoto T,
Kobayashi T, Kamata
K. Relationship between peroxisome proliferator-activated
receptors (PPAR alpha and PPAR gamma) and
endothelium-dependent relaxation in
streptozotocin-induced diabetic rats
(2003) Br J Pharmacol 140: 23-32.
12. Matsumoto T, Ishida K, Nakayama N, Kobayashi
T, Kamata K. Involvment of NO and
MEK/ERK pathway in
enhancement of endothelin-1-induced mesenteric artery contraction
in later-stage type 2 diabetic Goto-Kazizaki rat (2009) Am J Physiol Heart Circ
Physiol 296: H1388-97.
13. Ergul A. Endothelin-1 and diabetic
complications: focus on the vasculature. (2011) Pharmacol Res 63: 477-482.
14. Pernow
J, Shemyakin A,
Böhm F. New
perspectives on endothelin-1
in atherosclerosis and diabetes mellitus. (2012) Life Sci 91: 507-516.
15. Jörneskog
G, Kalani M,
Kuhl J, Båvenholm
P, Katz A,
et al. Early microvascular dysfunction in healthy
normal-weight males with heredity for type 2 diabetes. (2005) Diabetes Care 28:
1495-1497.
16. Tooke JE. Capillary pressure in
non-insulin-dependent diabetes (1983) Int Angiol 2: 167-171.
17. Tooke JE Microvascular haemodynamics in
diabetes mellitus. (1986) Clin Sci (Lond) 70: 119-125.
18. Boulton AJ, Scarpello JH, Ward JD. Venous
oxygenation in the diabetic neuropathic foot: evidence of arteriovenous
shunting? (1982) Diabetologia 22: 6-8.
19. Fagrell B, Jörneskog G, Intaglietta M.
Disturbed microvascular reactivity and shunting - a major cause for diabetic
complications. (1999) Vasc Med 4: 125-127.
20. Noda K, Nakao S, Ishida S, Ishibashi T.
Leukocyte adhesion molecules in diabetic retinopathy. (2012) J Ophthalmol 2012:
279037.
21. Romano
C, Tamburella C,
Costa M, Messina
M, Fassari AL,
et al.Aminaphtone therapy
in patients with type 1 diabetes and albuminuria: a case report. (2014) J
Med Case Rep 8: 443.
22. Romano C, et al. Preliminary findings about
effectiveness of aminaphtonetherapy in diabetic
microangiopathy. Accepted on Journal of Endocrinologyand Diabetes
Research, 2015.
23. Lenna S, et al. Novel mode of action of the
aminaphtone: down-regulation of E-selectine
expression in ECV 304 cells (2006) Int Angiology 25: 189.
24. Scorza R, Santaniello A, Salazar G, Lenna S,
Colombo G, et al. Aminaftone, a derivative of
4-aminobenzoic acid, downregulates endothelin-1 production in ECV304 Cells: an
in vitro Study (2008) Drugs R D 9: 251-257.
25. Scorza R, Santaniello A, Salazar G, Lenna S,
Della Bella S, et al. Effects of aminaftone
75 mg TID
on soluble adhesion
molecules: a 12-week, randomized,
open-label pilot study in patients with systemic sclerosis (2008) Clin Ther 30:
924-929.
26. Scorza R, et al. Aminaftone enhances
iloprost beneficial effects in patients with systemic
sclerosis and recurrent
ulcers (2009) ACR/ARHP
Annual Scientific Meeting –
Philadelphia, PA, October 16-21.
27. Matsumoto
T, et al.
Linking the beneficial
effects of current
therapeutic approaches in
diabetes to the
vascular endothelin system.
(2014) Life Sciences 118:
129-135.
28. Kohan DE, Pollock DM. Endothelin antagonists
for diabetic and non-diabetic chronic kidney
disease. (2013) Br J Clin Pharmacol 76: 573-579.
29. Mann JF, Green D, Jamerson K, Ruilope LM,
Kuranoff SJ, et al. A . Mann JF, Green
D, Jamerson K, Ruilope LM, Kuranoff SJ, et al. Avosentan for overt diabetic
nephropathy. (2010) J Am Soc Nephrol 21: 527-535.
Keywords
Diabetes, Diabetic vasculopathy


 PDF
PDF