Research Article :
Daphna R Atar-Zwillenberg, Michael Atar, Gianni Morson and Udo M Spornitz In
principal, three endocrinesystems are involved in the regulation of carbohydrate metabolism in
amphibians: the pancreatic system, the thyroid hormones and the
adenohypophysis-interrenal axis [1]. Although this holds also true for mammals
and fish, many pronounced species specificities make it rather unlikely, that
development of carbohydrate regulation in vertebrates followed a phylogenetic
stringent scheme. Each of the classes of vertebrates has developed different
mechanisms for the regulation of carbohydrate metabolism, and major differences
exist already within different species of each class [2]. Therefore, hormones
exert many different effects on the carbohydrate metabolism in amphibians and
mammals. Because of the typical life cycle of amphibians which includes a
metamorphosis from larvae or tadpoles to adult animals, the stage-specific
regulation of carbohydrate metabolism is an important prerequisite for normal amphibian
development. Hormones like insulin,
glucagon and thyroxine work together with corticosteroids to regulate the
glycogen levels in liver and muscle and/or the blood glucose level in a very
stage-specific manner [3]. It has been shown that the same hormones may not
only act in different ways but also exert opposite effects during different
developmental stages of amphibian animals [4-6]. However, the precise way in
which this stage-specific regulation takes place and how the involved hormones
interact with each other is still poorly understood. In this study, the South
African clawed toad Xenopuslaevis (Daudin), an anuran amphibian, was taken as a model organism to
investigate the hormonal regulation of glycogen metabolism in adult amphibians,
because it was known from former studies to have rather stable glycogen content
as found under various experimental conditions [7]. Under
physiological conditions the liver
glycogen content of adult Xenopus
laevis toads ranges between 10 and 20% of the liver wet weight [8]. In
naturally occurring populations of Xenopus
laevis this value may be influenced to a great extent by a number of
different environmental factors, such as seasonal changes, temperature, or food
supply, as well as by the age and sex (gender roles) of the animals [9].Preliminary
investigations of the glycogen metabolism in the adult toad have shown that
prolonged starvation (up to 60 days), exposure to cold (1° C-4°
C) and application of various hormones did not cause the glycogen content
of the Xenopus liver to be reduced to
values below 10% [10].It was the aim of this study to
analyze systematically the effects of hormones and various substances relevant
to the glycogen/glucose balance in adult Xenopus
toads of both sexes. In addition, we examined ultrastructural changes in the
hepatocytes induced by the different treatments. We also intended to elucidate
the regulating mechanisms of liver glycogen turnover by histochemical detection
of glycogen-relevant enzymes. Materials If not cited otherwise, the
chemicals used were products of Merck and Fluka and were of analytical grade.
Buffer solutions were always prepared with tri-distilled water. Animals Many of the male and female Xenopus laevis (Daudin), South African
clawed toads, were either purchased from a breeding colony maintained by Dr. Ch
H Thiebaud of the Institute for Experimental Zoology, University of Geneva
(Switzerland), or were kindly donated by Prof. Dr. L Du Pasquier of the Basel
Institute for Immunology
(Basel, Switzerland). The majority of the animals used, however, were bred and
reared in our own laboratory. The normal table of Nieuwkoop and Faber (1975)
was used to determine the stages [11]. The animals were maintained in large
plastic tanks with filtered tap water which was at 18° C - 20° C. The animals
were kept under a light regimen of approximately 12-hr light/12-hr dark. The
toads were fed chopped beef heart twice a week. The 2-4 years old animals
weighed 30-50 g (males) and 60-90 g (females). One day before the start of each
experiment feeding was stopped in order to eliminate dietary variations.
Experiments were done with male and female groups of five animals each. The
experiments were carried out in accordance with the guidelines of the Swiss
Animal Care Decree, and were approved by the Cantonal Veterinary Office of
Basel, Switzerland. Hormonal
treatments and biochemical analyses The concentration for each
hormone or substance is indicated in table 1. A volume of 0.1-0.5 ml of the
hormone suspension (in 0.72% NaCl) was injected into the dorsal lymph sac of
each animal. Control animals were injected with 0.1 ml of 0.72% NaCl alone. The
duration of exposure and the number of injections are summarized in (Table 1). Before anesthesia by
immersion in 1% MS-222 the toads were injected 1250 I.U. Liquemin
(Heparin). Blood was collected by cardiac puncture, mixed immediately with 3.5%
sodium citrate and centrifuged for 15 min at 3000 rpm. The supernatant was
stored at 4°C prior to analysis. Liver and muscle glycogen contents were
determined biochemically according to the method of Roe and Dailey (1966) [12].
The blood glucose was measured according to Schmidt (1961) using the glucose
determination kit from Boehringer, Mannheim, Germany [13]. Blood lipids were
determined according to Trinder (1969) using the lipid determination kits from
Boehringer, Mannheim, Germany [14]. Table 1: Hormonal
treatments, hormone doses and duration of experiments. Electron
microscopy Samples of hepatic tissue and
hind leg muscle were immersed into ice cold fixative (1% OsO4 in
Soerensen phosphate buffer at pH 7.2 and 175 mOsmol). Fixation was carried out
for 2 hr. After acetone dehydration the specimens were embedded in Epon. After
polymerization 1.5 µm semithin sections were cut with glass knives and stained
with 1% p-phenylendiamine for phase contrast microscopy. 60-90 nm ultrathin
sections were cut with diamond knives and stained with 5% aqueous uranyl
acetate and lead citrate [15]. Sections were examined with a Philips EM CM 100
operated at 80 kv. Images were digitally recorded and processed using the
software Analysis (Soft Imaging System GmbH, Münster, Germany). Histochemistry Liver samples were frozen in
liquid nitrogen and were stored at -80° C before use. 10 µm thin cryosections
of unfixed material were cut at a cryostat temperature of -20° C on a cryostat
(MICROM GmbH, Walldorf, Germany), thawed on cover slips, and air dried for
several minutes. Glucose-6-Phosphatase
(G-6-Pase): After 5 min of air drying at room
temperature, the sections were incubated in a medium containing lead nitrate as
modified by Maly and Sasse (1983) [16,17]. After incubation and rinsing, the
sections were mounted in glycerol jelly. Glycogen
phosphorylase: Glycogen phosphorylase activity was tested in sections which were air dried for 10
min after 1 h incubation (37° C) in a medium described by Takeuchi and Kuriaki
(1955) and modified by Lindberg and Palkama (1972) [18,19]. After incubation,
the sections were rinsed and stained in a sucrose-Lugol solution (10:1).
Mounting was carried out in Lugol-glycerol jelly (2:5).
The enzyme activity was quantified by semiquantitative evaluation of the
staining intensity, a method which despite its shortcomings seemed to be
justified, since all the examined slides were prepared under identical
conditions. Thus any general mistake should affect controls as well as
experimental preparations. Glyceraldehyde-3-Phosphate
dehydrogenase:The activity of this NAD-dependent
cytosolic enzyme was demonstrated by the methods of Henderson (1976) and De
Schepper, et al. (1985) as modified by P. Maly (personal communication).
Mounting was carried out in Mowiol [20,21]. Statistical
analysis The statistical significance of
our results was ascertained by a two-tailed Students t-test. The values of 5
independent replicates were expressed as mean ± the Standard Error of the Mean
(SEM), as indicated in the figures. Biochemical
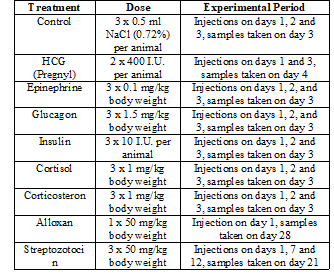
analyses Liver
glycogen: The biochemical determination of the Xenopus liver glycogen showed that, with
one exception, the glycogen content was neither reduced nor augmented
considerably through the application of insulin, glucagon, epinephrine,
cortisol, corticosterone, human chorionic gonadotropin, alloxan, or
streptozotocin, as compared to the controls. The mean values of the liver
glycogen contents of males ranged between 15% and 20% of the liver wet weight,
whereas the liver glycogen contents of females ranged between 10% and 15%. We
detected a slight reduction in the glycogen contents of males treated with
alloxan, but this difference was not statistically significant. Only human
chorionic gonadotropin which induces the vitellogenic response resulted in
a marked decrease of the liver glycogen stores in females. Their glycogen
content dropped to less than 5% as compared to about 15% in control females.
Contrasting this, the male liver glycogen content remained either stable or was
even increased to a certain extent (Figure
1). Muscle glycogen: The analysis of the
muscle glycogen content did not reveal significant differences between the
different hormonal treatments and the controls in Xenopus males and females (Figur
2). The mean values of the muscle glycogen contents of males ranged between
0.35% and 0.45% of the muscle wet weight, whereas the muscle glycogen contents
of females ranged between 0.25% and 0.32%. Only alloxan treated males showed a
slightly reduced muscle glycogen content, paralleling the findings for the
liver glycogen content. Blood glucose: The biochemical
detection of blood glucose showed significant differences in blood sugar
levels, not only between the different hormone applications and the controls, but
also between the sexes (Figure 3).
The average blood glucose level in control animals was about 50 mg/dl in males
and about 40 mg/dl in females. In males, this value remained unchanged after
application of insulin, glucagon, alloxan, HCG, or streptozotocin. Only the injections of either epinephrine or
cortisol resulted in an elevated blood glucose level, which however was only
significant with epinephrine. The blood
glucose level was in this case more than doubled. In females, insulin
resulted in a marked decrease, and glucagon in a slight increase of the blood
glucose concentration. Epinephrine (three times) and cortisol (two times)
drastically elevated the blood glucose levels in females. Alloxan, HCG, and
streptozotocin had no effect. Note:
* (** / ***) significantly different from control at p £ 0.05 (0.01/ 0.001). Blood lipid: The average level of blood triglycerides
in control animals was about 15 mg/dl in males and about 18 mg/dl in females.
The male blood lipid levels were only changed by insulin, epinephrine and cortisol,
where triglycerides tended to be elevated. Unfortunately, these results were
not found to be significant due to the rather high Standard Errors of the Mean.
In females, insulin slightly decreased the blood triglycerides, whereas
epinephrine, cortisol and streptozotocin
increased the lipid level. However, only the mean value of the cortisol treated
females proved to be significantly different from the controls (Figure 4). Note:
*significantly
different from control at p £ 0.05. Effects
of hormones on the ultrastructure of hepatocytes Light microscopic examination of
p-phenylendiamine stained semithin sections showed that the liver parenchyma of
Xenopus laevis was composed of cords
of several polyhedral cells. The nuclei of the hepatocytes usually occupied the
pole opposite from the bile canaliculus. Except for some basophilic substances
consisting of RER cisternae, dictyosomes of the Golgi complex, and mitochondria
in the cell periphery and in the peribiliary region, most of the cytoplasm was
occupied by an amorphous, dark material which by ultrastructural examination
was determined to be glycogen. We also observed large pigment aggregates
embedded in the liver parenchyma. By examination of semithin sections we detected only slight differences in the
cellular morphology of the hepatocytes taken from differentially treated Xenopus males and females. The primary
purpose of this technique was to give a survey of the unusual structural
organization of the Xenopus liver
parenchyma (Figure 5). Electron
microscopic examination of hepatocytes taken from Xenopus laevis male controls showed an ultrastructural picture
which was characterized by its most prominent feature, the large amount of
glycogen deposits. The hepatocytes contained more or less evenly distributed
glycogen particles of the adult alpha type forming typical rosettes. The cells
also contained some lipid droplets which were mostly present in groups of three
to five individual droplets. Occasionally, some small and rounded mitochondria
were visible at the periphery of the hepatocytes. The parenchymal liver cells of
male controls showed few signs of synthetic activity. This was reflected by the
small amount of endoplasmic reticulum and of dictyosomes in these cells. The
nuclei sometimes showed indentations and contained a lightly staining nucleolus.
Surface invagination and densely packed chromatin indicated that these nuclei
were metabolically inactive, showing limited production of nuclear
ribonucleic acid (Figure 6). In
general, the morphology of hepatocytes in Xenopus
female controls was very similar to the ultrastructure of male cells. However,
we found more cell organelles in the female liver cells, indicating a higher
level of activity (Figure 7). In
general, the morphology of parenchymal liver cells was neither significantly
changed by the pancreatic hormones nor by the diabetes inducing substances
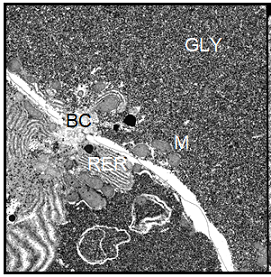
alloxan and streptozotocin as compared to the cells from controls. Note: BC=Bile
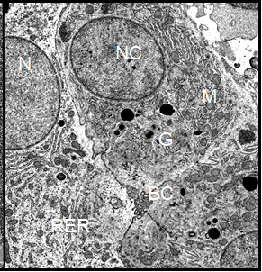
Canaliculus, G=Golgi apparatus, GLY=Glycogen, L=Lysosomes, M=Mitochondrion,
RER=Rough Endoplasmic Reticulum, LD=Lipid Droplet, N=nucleus. The hepatocytes of insulin
treated males contained slightly more glycogen than the cells of control males.
Except for the lipid droplets, the content of all other cell components was
slightly decreased, indicating that the synthetic activity in these cells was
reduced. In contrast, the insulin treatment had little if any effect on the
ultrastructure of female hepatocytes. The parenchymal
liver cells of glucagon treated toads showed only few inconspicuous
differences compared to the control. The cells still contained large areas
filled with glycogen particles, and the amount of cell organelles reflected a
basic level of cellular activity. Thus, the ultrastructural observation well
supported our biochemical data, according to which the liver glycogen content
was not decreased by the glucagon treatment. In Xenopus toads treated with alloxan, slightly less glycogen was
present than in the controls, again paralleling our biochemical data. The
hepatocytes from streptozotocin treated toads showed no signs of an altered
ultrastructure. It appears that neither the toxicity of the treatment nor the
resulting diabetes mellitus was able to affect the morphology of Xenopus hepatocytes significantly. In
contrast to the treatments described above, epinephrine clearly affected the
ultrastructural picture in hepatocytes from Xenopus
males and females. Whereas the liver glycogen content remained unchanged by the
treatment, the amount of cell components involved in the protein synthetic
machinery was strongly increased. Also the cell nucleus gave the
impression of increased activity, since the nuclear chromatin was less densely
packed than in the controls; it appeared finely dispersed within the nucleus.
In the parenchymal liver cells from cortisol treated toads we found the same
characteristic morphological features as in those from the controls, i.e. high
glycogen content and a basic level of cellular activity. In male Xenopus toads treated with human
chorionic gonadotropin, the morphology of the hepatocytes was dominated by the
very high content of glycogen particles. Apart from that, no visible morphological
changes had occurred in these cells (Figure
8). In contrast, we detected multiple dramatic effects of the HCG treatment
on the female hepatocytes. The amount of stored glycogen was significantly
decreased. The most prominent feature of these cells was the considerable
proliferation of the granular
endoplasmic reticulum and an increase of all cell components involved in
protein synthesis. The cell nucleus showed a perfectly rounded shape and contained
dispersed chromatin (Figure 9). Histochemistry Glucose-6-Phosphatase
(G-6-Pase): In control males and females we observed
only a moderate G-6-Pase activity. In the insulin treated toads of both sexes,
however, the histochemical reaction was clearly reduced, whereas the glucagon
treated animals showed a slight increase in G-6 - Pase activity. In the
hepatocytes from toads treated with alloxan, G-6-Pase activity was strongly
elevated. We also detected a clearly increased enzyme activity in epinephrine
treated Xenopus, as well as in the
cortisol treated toads. G-6-Pase activity remained unchanged in HCG treated
males (Figure 10), but the hormone
treatment apparently had a strongly enhancing effect on the enzyme activity in
the female hepatocytes (Figure 11).
The treatment with streptozotocin caused no visible differences in the G6Pase
activity as compared to the control animals. Glycogen
phosphorylase: The histochemical analysis of the
glycogen phosphorylase activity showed that, with the exception of HCG, the
enzyme activity was generally much higher in males than in females. However, we
detected no significant changes in the histochemical reactions from toads
treated either with the pancreatic hormones or with the diabetes inducing
substances alloxan and streptozotocin as compared to the controls. The enzyme
activity was strongly enhanced in epinephrine treated males but not in the
female toads, where the reaction was only slightly elevated. A similar picture
was observed in cortisol treated animals, namely a clearly increased glycogen
phosphorylase activity level in the male toads, and only a slightly elevated
activity in the females. In males treated with HCG, the histochemical reaction
was diminished, whereas in the HCG treated females a slightly elevated enzyme
activity was detected. While the hormonal regulation of
the entire energy metabolism in mammals is well understood, there are
controversial facts known about how hormones influence the carbohydrate
metabolism in amphibians. Therefore, it was the main purpose of the present study
to analyze the effects of various hormones on the glycogen/glucose balance in Xenopus as an important species of the
anurans. Our results gained from the biochemical and histochemical analyses as
well as from the ultrastructural examinations clearly showed that major
differences indeed exist between the effects in mammals and those in
amphibians. While in mammals a strong influence of the pancreatic hormones on
carbohydrate metabolism has been described these hormones only weakly affected
the glycogen/glucose balance in Xenopus
[22,23]. The liver glycogen content was neither significantly augmented by
insulin nor reduced by glucagon, and the blood glucose level was significantly
affected only in insulin treated females, where the concentration was decreased. The diabetes inducing substances
alloxan and streptozotocin likewise showed no significant changes in the
glycogen/glucose balance of the treated animals. It has been shown earlier in
our laboratory that larval as well as young postmetamorphic
Xenopus toads were influenced by
the pancreatic hormones in a manner comparable to the reaction in mammals [10].
Therefore, we assume that the receptors for pancreatic hormones are either
deactivated or no longer expressed with the onset of sexual maturity. In contrast
to the weak responses to pancreatic hormones, we found strong effects of
epinephrine and cortisol on the glycogen/glucose balance in Xenopus. While the liver and muscle
glycogen contents were not affected by the treatments, we detected an increase in
the blood glucose concentration as well as elevated blood lipid levels in both
sexes. The lacking response of liver and muscle glycogen to epinephrine,
cortisol and corticosterone might reflect indeed a non-responsiveness of
hepatic carbohydrate metabolism in this species. It might, however, be just as
well that it reflects a systemic counterbalance reaction, e.g. through massive gluconeogenesis.
In vivo experiments with Xenopus
hepatocytes showed a marked response to epinephrine [2]. The same authors did,
however, not investigate any of the other hormones used in our study. So one
might speculate that the missing systemic regulation leads to a persisting
expression of hormone receptors in cultured hepatocytes. Our histochemical investigations
revealed an increased G-6-Pase activity in epinephrine and cortisol treated
males and females, an enhanced glycogen phosphorylase activity in males only,
and a slightly elevated glyceraldehyde-3-phosphate dehydrogenase activity in
females. On the ultrastructural level, the protein synthetic activity was
strongly enhanced in epinephrine treated Xenopus
toads of both sexes. From the finding that the blood glucose concentrations
were elevated in epinephrine and cortisol treated toads without the liver and
muscle glycogen contents being affected, the question arises as to where the
additional blood glucose came from. By the histochemical reactions described
above, we have tested the obvious possibility that gluconeogenesis was
occurring. It is, however, not yet fully
understood how these hormones control gluconeogenesis in amphibia. In Xenopus females treated with HCG, we
observed a classic induction of the so called vitellogenic response which
resulted in a marked reduction of the liver glycogen stores as well as in a
drastically altered ultrastructure of the hepatocytes. It has been shown by several
authors that vitellogenin, the egg yolk precursor protein, is synthesized in
the hepatocytes, secreted into the bloodstream, and transported to the ovaries
during the vitellogenic period [26-28]. The cytological changes observed in our
TEM micrographs reflect the enhanced vitellogenin synthesis. In the HCG treated
males, we detected neither a decrease in the liver glycogen content nor
morphological changes in the hepatic ultrastructure. These findings suggest
that the response to HCG described in females may be an indirect effect of this
hormone by stimulation of ovarian estrogen secretion. Our suggestion is
strongly supported by Nicholls, et al. (1968) who observed that injection of
pregnant mare serum gonadotropin caused a vitellogenic response in intact Xenopus females but not in males or
ovariectomized females [27]. Since Xenopus tadpoles and juveniles do react to hormonal stimulation the
missing response in the adult animal certainly needs further investigation [3,10].
Pure counterbalance of glycogenolysis by gluconeogenesis alone cannot account
for the glycogen stability. This would imply a high turnoverrate for glycogen.
Glycogen as such does, however, not just disappear by means of dissolution. In
mammals, at least, its turnover involves the proliferation of smooth
endoplasmic reticulum [29]. So we believe that glycogen in Xenopus hepatocytes is stable on the basis of a deactivation or
missing expression of the hormone receptors. Summary
and Conclusions To summarize the results presented in this paper, we found that the liver
glycogen content in Xenopus laevis is
very high and is not easily degraded by hormone application. The only situation
where glycogen is significantly reduced is in females during HCG stimulated
vitellogenesis. Since we assume that this effect is indirect and dependent on
the action of estrogen, we suggest that this response might be specific for
estrogens. The question of the meaning of this extreme liver glycogen stability
arises, and what the importance of glycogen as an energy storage and reserve
substance might be. From our data we conclude that in Xenopus laevis, the liver glycogen is apparently not the primary
source for the glucose need of several tissues as is the case in mammals. Since
the blood glucose levels in epinephrine and cortisol treated toads were
elevated without the corresponding liver and muscle glycogen contents being
affected, the additional glucose must have come from other sources. Our histochemical results strongly suggest that gluconeogenesis
from protein and/or lipid precursor substrates may be involved in the
regulation of the glycogen/glucose balance in Xenopus. We propose that the preservation of high glycogen levels
in favor of lipid or protein metabolism must be understood in the context of
the amphibian lifestyle as oviparous and poikilothermic animals. The rapid
mobilization of glycogen stores in Xenopus
females during vitellogenesis apparently takes place in order to provide the
carbohydrate supply in the oocytes. We believe that this process is a crucial
prerequisite for the survival of this anuran species, since the embryo must
live on the deposited energy reserves for a long time period. Another factor
playing a role in the unusual glycogen metabolism in Xenopus is the ability of these poikilothermic amphibians to
decrease their rate of glucose production under inconvenient conditions. In our
opinion, this ability constitutes a fundamental difference between the
homeothermic mammals and the poikilothermic amphibians which allows the latter
to utilize relatively slow processes such as the transformation of protein and
lipid to glucose as primary sources of glucose production. Moreover Xenopus laevis belongs to the group of
amphibians which do not hibernate, but passes through a period of aestivation,
which may also play a role when comparing different families of amphia with
respect to carbohydrate metabolism. 1.
Hanke W and Neumann U.
Carbohydrate metabolism in amphibian (1972) Gen Comp Endocrinol Suppl 3:
198-208. 2.
Ade T, Segner H and Hanke W.
Hormonal response of primary hepatocytes of the clawed toad, Xenopus laevis (1995) Exp Clin
Endocrinol Diabetes 103: 21-27. https://doi.org/10.1055/s-0029-1211325
3.
Hanke W. Die hormonale Regulation
des Stoffwechsels bei Amphibien (1974) Fortschr Zool 22: 431-455. 4.
Gray KM and Janssens PA. Gonadal
hormones inhibit the induction of metamorphosis by thyroid hormones in Xenopus laevis tadpoles in vivo, but not
in vitro (1990) Gen Comp Endocrinol 77: 202-211. https://doi.org/10.1016/0016-6480(90)90304-5
5.
Hanke W and Leist KH. The effect
of ACTH and corticosteroids on carbo-hydrate metabolism during the
metamorphosis of Xenopus laevis
(1971) Gen Comp Endocrinol 16: 137-148. https://doi.org/10.1016/0016-6480(71)90216-4
6.
Wong KL and Hanke W. The effects
of biogenic amines on carbohydrate metabolism in Xenopus laevis Daudin (1977) Gen Comp Endocrinol 31: 80-90. https://doi.org/10.1016/0016-6480(77)90194-0
7.
Spornitz UM. Studies on the liver
of Xenopus laevis, 1-The
ultrastructure of the parenchymal cell (1975) Anat Embryol 146: 245-264. https://doi.org/10.1007/bf00302173
8.
Spornitz UM. Studies on the liver
of Xenopus laevis, III-The
ultrastructure and the glycogen content of the developing liver (1978) Anat
Embryol 154: 1-25. https://doi.org/10.1007/bf00317951
9.
Merkle S. Sexual differences as
adaptation to the different gender roles in the frog Xenopus laevis Daudin (1989) J Comp Physiol B 159: 473-480. https://doi.org/10.1007/bf00692419
10. Spornitz
UM and Morson G. Sex- and age-dependent hepatic glycogen stability in Xenopus laevis (1992) Acta Anat 143:
168. 11. Normal
Table of Xenopus laevis (Daudin),
Nieuwkoop PD and Faber J (Eds.) (1975) North-Holland, Amsterdam. 12. Roe
JH and Dailey RE. Determination of glycogen with the anthrone reagent (1966)
Anal Biochem 15: 245-250. https://doi.org/10.1016/0003-2697(66)90028-5
13. Schmidt
FH. Enzymatic determination of glucose and fructose simultaneously (1961) Klin
Wochenschr 39: 1244. 14. Trinder
P. The Effect of Nutritional Lipid Supplementation on Serum Lipid Levels and
Effectiveness of Antitubercular Chemotherapy (1969) Ann Clin Biochem 6: 24. 15. Reynolds
ES. The use of lead citrate at high pH as an electron-opaque stain in electron
microscopy (1963) J Cell Biol 17: 208-213. https://doi.org/10.1083/jcb.17.1.208
16. Chiquoine
AD. The distribution of glucose-6-phosphatase in the liver and kidney of the
mouse (1953) J Histochem Cytochem 1: 429-435. https://doi.org/10.1177/1.6.429
17. Maly
IP and Sasse D. A technical note on the histochemical demonstration of G6Pase
activity (1983) Histochemistry 78: 409-411. https://doi.org/10.1007/bf00496628
18. Takeuchi
T and Kuriaki H. Histochemical detection of phosphorylase in animal tissues
(1955) J Histochem Cytochem 3: 153-160. https://doi.org/10.1177/3.3.153
19. Lindberg
LA and Palkama A. The effect of some factors on the histochemical demonstration
of liver glycogen phosphorylase activity (1972) J Histochem Cytochem 20:
331-335. https://doi.org/10.1177/20.5.331
20. Henderson
B. Quantitative cytochemical measurement of glycer¬aldehyde 3-phosphate
dehydrogenase activity (1976) Histochem 48: 191-204. 21. De
Schepper GG, Van Noorden CJ and Koperdraad F. A cytochemical method for
measuring enzyme activity in individual preovulatory mouse oocytes (1985) J
Reprod Fertil 74: 709-716. https://doi.org/10.1530/jrf.0.0740709
22. Eckert
R and Randall JD. Tierphysiologie (1993) Georg Thieme Verlag, Deutschland 724. 23. Shafrir
E, Bergman M and Felig P. The Endocrine Pancreas: Diabetes Mellitus (2nd Edn)
Felig P, Baxter JD, Broadus AE and Frohman LA (Eds.) (1987) Endocrinology and
Metabolism, McGraw-Hill Book Company, USA. 24. Janssens
PA. Interference of metyrapone with the actions of cortisol in Xenopus laevis Daudin and the laboratory
rat (1967) Gen Comp Endocrinol 8: 94-100. https://doi.org/10.1016/0016-6480(67)90117-7
Woof C and Janssens PA. Effects of fasting and cortisol administration on
carbohydrate metabolism in Xenopus
laevis Daudin (1978) Gen Comp Endocrinol 36: 346-359. https://doi.org/10.1016/0016-6480(78)90116-8
25. Follett
BK, Nicholls TJ and Redshaw MR. The vitellogenic response in the South African
clawed toad (Xenopus laevis Daudin)
(1968) J Cell Physiol 72: 91-102. https://doi.org/10.1002/jcp.1040720408 26. Nicholls
TJ, Follett BK and Evennett PJ. The effects of oestrogens and other steroid
hormones on the ultrastructure of the liver of Xenopus laevis Daudin (1968) Zeitschrift für Zellforschung 90:
19-27. https://doi.org/10.1007/bf00496699
27. Wallace
RA and Dumont JN. The induced synthesis and transport of yolk proteins and
their accumulation by the oocyte in Xenopus
laevis (1968) J Cell Physiol 72: 73-101. https://doi.org/10.1002/jcp.1040720407
28. Cardell
RR. Smooth endoplasmic reticulum in rat hepatocytes during glycogen deposition
and depletion (1977) Int Rev Cytol 48: 221-279. https://doi.org/10.1016/s0074-7696(08)61746-5
29. Janssens
PA. Hormonal control of glycogenolysis and the mechanism of action of
adrenaline in amphibian liver in vitro (1983) Gen Comp Endocrinol 49: 477-484. Tel: +44 208 457 9080, E-mail: atardrs@yahoo.com Citation Atar-Zwillenberg RD, Atar M, Morson G and Spornitz MU. The role of hormones in
the regulation of glycogen metabolism in the clawed toad Xenopus Laevis
(Daudin) (2019) J Obesity and Diabetes 3: 17-24The Role of Hormones in the Regulation of Glycogen Metabolism in the Clawed Toad Xenopus Laevis (Daudin)
Abstract
The hormonal regulation of
amphibian glycogen metabolism was studied in Xenopus laevis as a typical member
of the anurans (tailless amphibians).The main focus of this study was given to
the effects of various hormones on the glycogen/glucose balance in adult toads.
We determined biochemically the liver and muscle glycogen contents as well as
the blood glucose and lipid levels for a number of hormones and also diabetes
inducing substances. Additionally, we examined ultrastructure changes in
hepatocytes induced by the various treatments, and also investigated the
activity of carbohydrate-relevant enzymes by histochemistry. With one
exception, the liver glycogen content of Xenopus remained basically unchanged
by the treatments or was even slightly enhanced. Only human chorionic
gonadotropin, through which the vitellogenic response is triggered, prompts a
significant decrease of liver glycogen in females. Under the same conditions
the male liver glycogen content remained stable. Muscle glycogen contents were
not affected by any of the treatments. Blood glucose and lipid levels on the
other hand were elevated considerably in both sexes after application of either
epinephrine or cortisol. The ultrastructural examination revealed a
proliferation of Rough Endoplasmic Reticulum (RER) in hepatocytes from
epinephrine treated toads of both sexes as well as from HCG treated females. By
histochemistry, we detected an elevated glucose-6-phosphatase activity in the
hepatocytes from toads treated with either epinephrine or cortisol. These
treatments also led to enhanced glycogen phosphorylase activity in males, and
to a slightly elevated glyceraldehyde-3-phosphate dehydrogenase activity in
females. Our results show that the hepatic glycogen is extremely stable in
adult Xenopus. Only vitellogenesis causes a marked utilization of glycogen.
Since the blood glucose levels are elevated in epinephrine or cortisol treated
toads without the liver glycogen being affected, we conclude that either
protein and/or lipid metabolism are involved in carbohydrate metabolism in
Xenopus laevis
Full-Text
Introduction
Materials
and Methods
Results
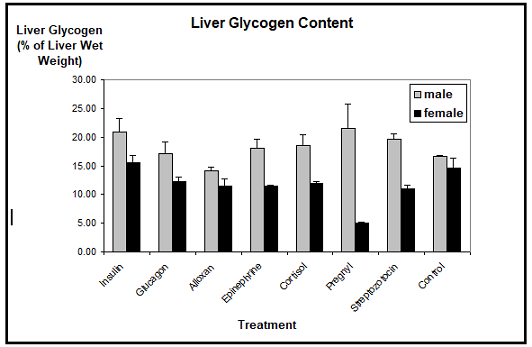
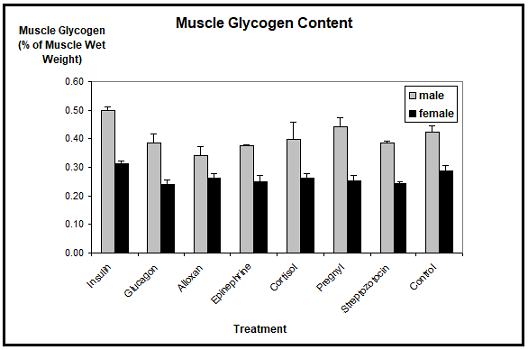
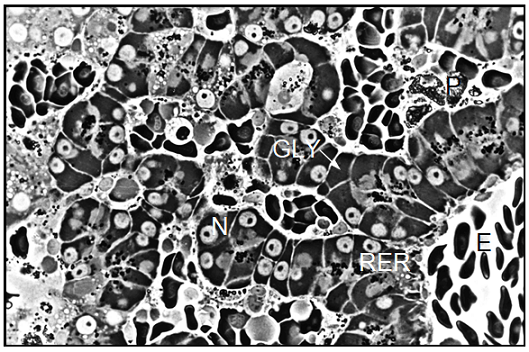
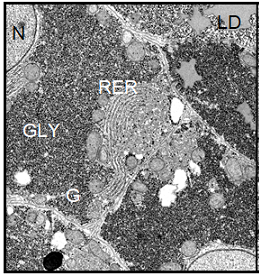
Figure 6:
(Left) Electron micrograph showing parenchymal liver cells from a Xenopus laevis control male. The
ultrastructural picture is characterized by the large amount of deposited
glycogen and represents a basic level of cellular and protein synthetic
activity. Magnification: ´ 4700.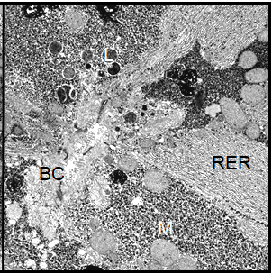
Glyceraldehyde-3-Phosphate dehydrogenase: The
histochemical reaction of glyceraldehyde-3-phosphate dehydrogenase was slightly
stronger in the hepatocytes from control males than in those of the females. In
epinephrine treated males, the enzyme activity was unchanged, but was slightly
elevated in females. In contrast, the reactions were slightly increased in the
hepatocytes from both cortisol treated Xenopus
males and females. Discussion
Our results suggest that the mechanisms responsible for the regulation of the
glycogen/glucose balance differ in Xenopus
males and females. Since the glycogen phosphorylase activity was enhanced in
the males without the glycogen content being reduced, we must assume that the
occurring glycogenolysis is compensated for by simultaneous glycogen synthesis,
and that the liver glycogen turnover rate is generally increased in epinephrine
and cortisol treated males. In the females, we detected a strongly enhanced
G-6-Pase activity which apparently was not correlated to increased
glycogenolysis. In addition, the activity of glyceraldehyde-3-phosphate
dehydrogenase was slightly elevated in epinephrine and cortisol treated female
toads, which is also indicative of enhanced gluconeogenesis. From this data, it
seems reasonable to conclude that gluconeogenesis does occur at least in female
toads upon stimulation with epinephrine and cortisol. Also other authors have
discussed the possibility that conversion of protein and lipids to glucose may
take place in amphibia under the influence of catecholamines and
corticosteroids [1,5,24,25].
In the attempt to evaluate the results presented in our paper it appears to be
critical to differentiate well between different species of amphibia. Results
obtained from experiments with a member of the Ranidae family for instance
differ in many respects from results obtained from species of the family of
Pipidae like Xenopus, a fact which is
clearly demonstrated by a number of investigations [3,30]. Acknowledgements
We wish to thank Dr. P. Maly (Institute of Anatomy, Basel) for advice and
assistance with the histochemical assays, and Prof. Dr. L. Du Pasquier (Basel
Institute for Immunology) for kindly providing many of the Xenopus toads used in this study. References
Corresponding
author: Daphna R Atar-Zwillenberg, Sweet Smile
LLP, Crest Cottage, The Crest London NW4 2HN, United Kingdom,
Keywords
Xenopus,
Hepatocytes, Glycogenolysis, Gluconeogenesis, Ultrastructure, Hormonal treatment.