Research Article :
The susceptibility to frequent and protracted infections in diabetics
is a well-known clinical pathologic event, following large blood vessel
complications as atherosclerosis, ischemic heart disease, renal failure and
cerebrovascular accidents as major causes of death [1]. However, the basis of
immunodeficiency and susceptibility to infections in diabetes are not
completely understood but pathophysiology studies seem include many mechanisms
involved in the diabetes immunodepression as deficit of lymphocytes response,
compromised function of neutrophil, humoral
immunity disorders, lower secretion of cytokines, angiopathy, increased
virulence of infectious microorganisms and apoptosis of leucocytes related to
hyperglycemia. These considerations took us to put under microscopic observations the
lymph-nodes of four patients with long-term insulindependent diabetes
mellitus, deceased for complications related to their main disease, with
reported clinical marks of microangiopathy as acquired blindness, renal failure
by glomerulosclerosis, renal necrotizing papillitis and diabetic retinopathy. Case 1 A
43 year old male, Physician, was hospitalized in December 2014 with acute
pneumonia, bilateral pleurisy, high fever and dyspnea. The patient progressed
rapidly into a coma and died without receiving treatment. His relatives
reported a history of IDDM diagnosed at 8 years of age. He was generally well
compensated with insulin treatment until age 35 when signs of renal failure and
hypertension appeared. Laboratory tests at that time showed a fasting plasma
glucose of <180 mg/dl, elevate creatinina at 3 to 4 mg/dl, proteinuria,
glycosuria and hypogammaglobulinemia. At age 40 he was subjected to ocular surgery for diabetic retinopathy. Later,
his temperament changed, he became irritable and refused appropriate treatment.
Hematological tests showed normal ranges of RBC and platelets and low WBC (2.7 to
3.2 X 109/L) with lymphocytopenia (15-20%). Autopsy examination showed
bilateral pneumonia with fibrinous pleurisy, severe diffuse nephrosclerosis, and
atherosclerosis with involvement of aorta, coronary arteries, and myocardiosclerosis. Case 2 A
65 year old man suffered from metabolic type1IDDM for 25 years. He was
hypertensive. Since age 58 he had been on hemodialysis after recurrent episodes
of pyelonephritis and subsequently necrotizing
papillitis. During a dialysis session the patient complained of acute
abdominal pain which was followed by intestinal hemorrhage. He was treated with
transfusion therapy but died in hypovolemic shock after three days. Prior to
his death laboratory tests had consistently shown elevated fasting glucose and
serum creatinina, severe anemia and leukocytosis with a marked lymphocytopenia
(≈6%). Autopsy examination demonstrated cholesterol
emboli of superior mesenteric arteries, multiple ulcerative and hemorrhagic
lesions involving the terminal ileum and right colon, severe atherosclerosis
with ulcerative plaques of the aorta, myocardiosclerosis and small myocardial
infarcts, bilateralrenal sclerosis from pyelonephritis and papillitis. Case 3 A
61 year old woman had metabolic type1 IDDM for 35 years and suffered several
episodes of pneumonia and pleurisy. She was hypertensive and had diabetic
retinopathy complicated by bilateral blindness at age 54. In
August 2015 she complained of sudden retrosternal pain for which she was
immediately hospitalized. The electrocardiogram and serum transaminase levels
documented a myocardial infarction. Her fasting serum glucose value was >500mg/dl
and hematological tests showed moderate anemia and leukocytosis with lymphocytopenia
(8%). She
was treated with fibrinolytc drugs and cardiokinetics, but on the 7th day after
admission hypotensive shock and death occurred.Autopsy examination showed a
large acute myocardial infarct involving the anterior area of the left
ventricle, severe atherosclerosis with thrombosis of the anterior coronary
artery, diffuse glomerulosclerosis,
areas of lung consolidation and pleural fibrosis.
Case 4 In
May 2016 a 51 year old man was found lifeless in the street and was immediately
transported to a trauma center where he was certified dead due to heart
fibrillation. His relatives reported that he had measles and chicken-pox at age
18 followed by IDDM. He was generally well compensated with insulin treatment,
although he suffered periodic hypoglycemic crisis. At age 44 he became
hypertensive, showing signs of renal failure (creatinina2.5-3mg/dl) and visual
deficit due to diabetic retinopathy. He suffered from chronic bronchitis and at
age 47 was hospitalized for bilateral pneumonia with pleurisy. At that time he
had a mild anemia with leukocytopenia
and lymphocytopenia (8-10%). Autopsy
examination showed severe atherosclerosis with ulcerative plaques, recent
thrombosis of the anterior coronary artery, myocardiosclerosis and some
cicatritial microinfarcts involving the left ventricle. Diffuse
glomerulosclerosis involved more than two-thirds of cortical tracts and
complete hyalinization of the pancreatic islets was evident.
Histology and Immunohistochemistry The
histological and immunohistochemical study was focused on lymph-nodes removed
during autopsies from axillary, supraclavicular, mediastinal and
retroperitoneal sites. All tissue samples were fixed in buffered formalin and
embedded in paraffin. Sections were stained with hematoxylin-eosin, Giemsa,
PAS, PAS-D, reticulin with Gomori procedure. Immunohistochemistry
was performed with a panel of monoclonal and polyclonal
antibodies listed in (Table 1),
with special target to endothelial cells (CD31, CD 34), follicular dendritic
cells (CD21 and CD35), B lymphoid cells ( L26, LN1, MB2 ), T lymphoid cells
(CD3, CD8, UCHL1, DF-T1, OPD4),monocytic cells (CD68 and KP1), interdigitating
dendritic cells (S 100 protein, PGM1). Technical methods were used according to
the LSAB 2 kit (Dakopatt,Carpinteria, CA, USA) and supersensitive biotin
streptavidin kit (Biogenex Laboratories, S. Ramon, CA,USA), visualization was
made with the immunoalkaline phosphatase or immunoperoxidase.
Dako, Dakopatts (Carpinteria, CA, USA, and Copenaghen, Denmark), Biogenex
Laboratories (San Ramon, CA, USA) (*). microwave pretreatment Table 1: Details of
antibodies used in the study. The
lymph nodes from each of the patients showed similar histopathologic changes. Lymphodepletion
of the B and T dependent areas was early apparent with rare small scattered
lymphoid follicles. The general architectural structure appeared modify by
presence of numerous vessels showing basement membrane thickening due to deposition
of homogeneous PAS+hyaline substance. Several of these vessels showed a
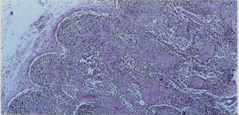
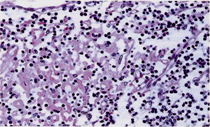
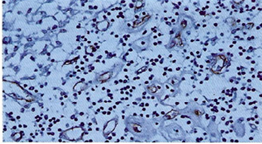
conglomerate appearance, and deposition of calcium microgranules (Figure 1a, Figure 1b). The cortical
and medullary sinuses were present, outlined by monolayered or hyperplastic
littoral cells. Diffuse depositions of hyaline substance were apparent especially
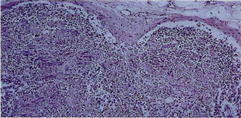
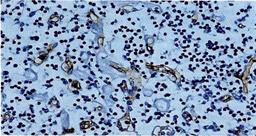
around small vessels and capillaries and highlighted by PAS and PAS-D (Figure 2). The thickened vessel walls were more clearly evident by immunostaining of the endothelial
cells with CD34 and CD31 in the cortex, paracortex and medullary sites (Figure 3a, Figure 3b). ln the
paracortical areas the number of T lymphocytes was very reduced as demonstrated
by UCHL 1, CD3, CD8 and OPD4. Depletion
of B
lymphocytes was apparent in the cortical and medullary areas using the L26 (CD20),
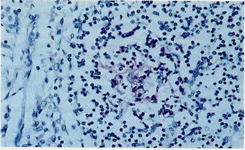
MB2 and LN1 (CDW75) antibodies. CD21 d CD35 could document a very low number of
dendritic reticulum cells among small B lymphocytes and few small cleaved cells
(Figure 4). S-100 protein revealed
several interdigitating dendritic cells while CD 68 (KP1) showed frequent
macrophages, generally inside the lymphatic vessels. The PCNA revealed a very
low lymphocyte proliferation index (5-10 %). Figure 1a: General view of
lymph-nodes showing structural modifications by diffuse hyaline substance,
marked lymphocytes depletion, absence of lymphoid follicles and deposition of
calcium microgranules. Hematoxylin-eosin stain (A60x, B 120x). Figure 1b: General view of
lymph-nodes showing structural modifications by diffuse hyaline substance,
marked lymphocytes depletion, absence of lymphoid follicles and deposition of
calcium microgranules. Hematoxylin-eosin stain (A60x, B 120x). Figure 2:Detail of
cortical area showing lymphocytes depletion along with deposition of hyaline
substance in capillary vessels. PAS-D 250x. Figure 3a:
lmmunohistochemistry using CD 34 highlights endothelial cells surrounded by
thickened hyaline substance together with severe lymphocytopenia (250 x). Figure 3b: lmmunohistochemistry
using CD 34 highlights endothelial cells surrounded by thickened hyaline
substance together with severe lymphocytopenia (250 x). Figure 4: Rarely it may be
possible observe, by immunohistochemistry, small aggregations of follicular dendritic
cells (red stain) but never complete lymphoid follicles. Generally cortical
sinuses (on the left) doesnt show modifications and are outlined by normal
littoral cells. (lmmunostaining for CD21 250x). Numerous
reports have demonstrated that patients with diabetes mellitus are more
susceptible to infections than non-diabetic individuals. Infections are a major
cause of morbidity and mortality in diabetic patients which may further have
ischemic heart disease, cerebro-vascular accidents, aortic aneurisms and renal
failure as diabetes complications. The immune defenses in diabetic individuals
have been investigated by in vitro studies of cells involved in immuniity. Abnormalities
have been shown in genetic (type 1), autoimmune (type 2) and metabolic (type 1and
2) IDDM although no conclusive data to explain the immune deficits have been
obtained. Among
patients with IDDM, HLA antigen serologic analyses indicate significant
positive associations with B8, B18, BW15, Dr3, Dr4, as well as other less
definable HLA associations [1]. Some combinations of these HLA antigen
expressions have been associated with T cell response abnormalities, decreased
antibody titers and C4 deficiency [2-5]. However,
defects of humoral immunity have also been reported in diabetics without any relation
to HLA phenotypes [6,7]. Abnormalities in immune response to infections in
diabetes mellitus, related to genetic factors, remain controversial. Autoimmunity
has been postulated as a cause of type 2 diabetes mellitus. As in other
autoimmune diseases immunodeficits may be associated with the primary disease.
Abnormalities of blood CD4/CD8 T lymphocyte subsets have been reported by
several authors as decreased, increased or normal [8]. The correlation of
CD4/CD8 ratio to infections or immunodeficiency in diabetes is presently
unclear. Indeed hyperglycemia may induce lymphopenia and lymphocyte subset
redistribution in selected reported clinical study [17]. Therefore In general
it is believed that a better regulation of diabetes mellitus leads to an
improvement the immune cells function and to a reduced risk of infection
complications [15]. Previously on the same line of research, MacCuish, et al.,
[18] examined the lymphocyte proliferation response to phytohemagglutinin in
type 1 diabetic patients and found inhibitionin
diabetics with poorly controlled disease (fasting glycemia>350 mg/dl )
compared with well controlled patients. Low plasma zinc levels have been
reported in type1 and type2 diabetes mellitus. The importance of zinc has been suggested because T cell function appears
related to thymulin which requires zinc to express its biological activities on
cellular
immunity. Zinc deficiency could therefore contribute in the lymphocyte
abnormalities described in diabetes and also on neutrophils (PMN) of Rhesus
monkeys [19-21]. Most likely metabolic defects and the other factors discussed above play an
important role, but sometimes studies are contradictory in the immune deficit
and propensity to infections in diabetic patients. In addition to these defects
impairment of leukocyte efficiency and diapedesis may be significant factors.
Thickening of capillary basement membranes observed by histomorphological
methods represents a specific modification related to the diabetes microangiopathy;
which may disrupt normal leukocyte egress. Basement Membrane Thickening (BMT) is characteristic of both major variants of
diabetes mellitus and it is believed to be the result of increased synthesis or
decreased degradation of basement membrane proteins [1].
However, because this membrane proves more permeable than normal, especially to
plasma proteins, insudation of proteins is thought to contribute to BMT. Microangiopathy
has been extensively described in the capillaries of renal glomeruli, retina,
skeletal muscles, skin, peripheral nerves, and other sites. The
lymph-nodes examined from several sites in each case always showed lymphodepletion
of T and B cell zones frequently between hyaline substance deposition and with cortical areas devoid of lymphoid
follicles (Figure 1,2,3). The explanation for the low number of DRCs may be traced to their origin. DRCs
are identifiable by immunohistochemistry using CD21 or CD35 MoAbs. They
represent a peculiar cell component of lymphoid follicles and are believed to
develop from local mesenchymal cells. Proposed origins have included
fibroblastic-like cells, mononuclear phagocytic cells [22-26], perivascular
cells and vessel endothelial cells. Previous studies seem to indicate that DRC may be derived from transformed
endothelial cells and constitute an enhancing microenvironment for B cell
lymphoid expansion [27-29]. Therefore, one cause of the paucity of lymphocytes
in diabetes mellitus could be related to lymph node microangiopathy. The involvement
of thin capillaries, besides compromising
the diapedesis
and the traffic of T and B cells could prevent the transformation of
endothelial cells into DRC, and eventually result in disruption of the normal
microenvironment for B cell lymphocyte expansion and differentiation with
failure of lymphoid follicles The diffuse involvement
of lymph nodes by diabetes microangiopathy as described in our four cases
perhaps could have a significant role in the immunodeficiency of IDDM. Likely
in the future the best treatment for diabetic patients could be on the strong
control of hyperglycemia and glycated hemoglobin with association of endothelial
protecting drugs [30]. 1.
Robbins
SL and Cotran RS. Pathologic Basis of Disease (1979) (2nd Edn)
Philadelphia-London Toronto, WB Saunders Company, London 327-343. 2.
Casqueiro
Ju, Casqueiro Ja and Alves C. Infections in patients with diabetes mellitus: A
review of pathogenesis (2012) Indian J Endocrinol Metab 27-36. https://doi.org/10.4103/2230-8210.94253
3.
Moutschen
MP, Scheen AJ and Lefebvre PJ. lmpaired immune responses in diabetes mellitus:
analysis of the factors and mechanisms involved. Relevance to the increased
susceptibility of diabetic patients to specific infections (1992) Diabete Metab
18: 187-201. 4.
McCombs
CC, Michalski JP, de Shazo R, Bozelka B and Lane JT. Immune abnormalitìes
associated with HLA-B8: lymphocyte subsets and functional correlates (1986)
Clin lmmunol lmmunophatol 39: 112-120. https://doi.org/10.1016/0090-1229(86)90210-2
5.
Ruben
FL, Fireman P, Laporte RE, Drash AL, Uhrìn M, et al. Immune response to killed
influenza vaccine in patients with Type1 diabetes: altered responses associated
with HLA-DR3 and DR4 (1988) J Lab Clin Med 112: 595-602. 6.
Verganì
D, Johnstone C, B-Abdullah N and Barnett AH. Low serum C4 concentrations:
inherited predisposition to insulin dependent diabetes (1983) Br Med J 286:
926-928. https://doi.org/10.1136/bmj.286.6369.926
7.
Ludwig
H, Eibl M, Schernthaner G, Erd W and Mayr WR. Humoral immunodeficiency to
bacterial antigens in patients with juvenile onset diabetes mellitus (1976)
Diabetologia 12: 259-262. https://doi.org/10.1007/bf00422093
8.
Pozzilli
P, Arduini P, Visalli N, Sutherland J, Pezzella M, et al. Reduced protection
against hepatitis B virus following vaccination in patients with type 1
(insulin-dependent) diabetes (1987) Diabetologia 30: 817-819. https://doi.org/10.1007/bf00275749
9.
Drell
DW and Notkins AL. Multiple immunological abnormalities in patients with Type
1(insulindependent) diabetes mellitus (1987) Diabetologia 30: 132-143.
https://doi.org/10.1007/bf00274217 10.
Delamaire
M, Maugendre D, Moreno M, Le Goff MC, Allanninic H, et al. Impaired leucocyte
functions in diabetic patients (1997) Diabet Med 14: 29-34. https://doi.org/10.1002/(sici)1096-9136(199701)14:1<29::aid-dia300>3.0.co;2-v
11.
Alexiewicz
JM, Kumar D, Smogorzewski M, Klin M and Massry SG. Polymorph nuclear leukocytes
in non-insulin-dependent diabetes mellitus: abnormalities in metabolism and
function (1995) Ann Intern Med 123: 919-924. https://doi.org/10.7326/0003-4819-123-12-199512150-00004
12.
Joshi
N, Caputo GM, Weitekamp MR and Karchmer AW. Infections in patients with
diabetes mellitus (1999) N Engl J Med 341: 1906-1912. https://doi.org/10.1056/nejm199912163412507
13.
Bertoni
AG, Saydah S and Brancati FL. Diabetes and risk of infection-related mortality
in the US (2001) Diabetes Care 24: 1044-1049. https://doi.org/10.2337/diacare.24.6.1044
14.
Shah
BR and Hux JE. Quantifying the risk of infectious diseases for people with
diabetes (2003) Diabetes Care 26: 510-513. https://doi.org/10.2337/diacare.26.2.510
15.
Pearson-Stuttard
J, Blundell S, Harris T, Cook DG and Critchley J. Diabetes and infection:
assessing the association with glycaemic control in population-based studies
(2016) The Lancet Review 4: 148-158. https://doi.org/10.1016/s2213-8587(15)00379-4
16.
Geerlings
SE, Hoepelman AIM. Immune dysfunction in patients with diabetes mellitus FEMS
Immunology and Medical Microbiology (1999) 26:259-265. https://doi.org/10.1111/j.1574-695x.1999.tb01397.x
17.
Von
Kanel R, Mills PJ and Dimsdale JE. Short-term hyperglycemia induces lymphopenia
and lymphocyte subset redistribution (2010) Life Sci 69: 255-262. https://doi.org/10.1016/s0024-3205(01)01127-4
18.
MacCuish
AC, Urbaniak SJ, Campbell CJ, Duncan LJP, and lrvine WJ. Phytohemagglutinin
transformation and circulating lymphocyte subpopulations in insulin-dependent
diabetic patients (1974) diabetes. Diabetes 23: 708-712. https://doi.org/10.2337/diab.23.8.708
19.
Niewoehner
B, Allen JI, Boosalis M, Levine AS, and Morley JE. Role of zinc supplementation
in type 2 diabetes mellitus (1986) Am.J Med 81: 63-68. https://doi.org/10.1016/0002-9343(86)90183-x
20.
Prasad
AS, Meftah S, Abdallah J, Kaplan J, Brewer GJ et.al. Serum thymulin in human
zinc deficiency (1988) J Clin lnvest 82: 1202-1210. https://doi.org/10.1172/jci113717 21.
Vruwink
KG, Fletcher MP, Keen CL, Golub MS, Hendrickx AG, et.al. Moderate zinc
deficiency in Rhesus monkeys an intrinsic defect in neutrophil chemotaxis corrected
by zincrepletion (1991) J Immunol 146: 244-249. 22.
Hensermann
U, Zurborn KH, Schroeder L and Stutte HJ. The origin of the dendritic reticulum
cell (1980) Cell Tiss Res
209:279-294. 23.
Mulier-Hermelink
HK. Gaudeker B, Drenckhahn D, Jaworsky K and Feldman C. Fibroblastic and
dendritic reticulum cells of lymphoid tissue (1981) J Cancer Res Clin Onco 101:
149-164. https://doi.org/10.1007/bf00405075
24.
Fliedner
A, Parwaresch MR. and Felier AC lnduction of antigen expression of follicular
dendritic cells in a monoblastic cell line-A contribution to its cellular
origin (1990) J Phat 161: 71-77. https://doi.org/10.1002/path.1711610112
25.
Soderstrom
N. Post-capillary venules as basic structural units in the development of
lymphoglandular tissue (1967) Scand J Haematol 4: 411-429. https://doi.org/10.1111/j.1600-0609.1967.tb01644.x
26.
Henry
K. Thymus, Lymph Nodes, Spleen and Lymphatics (1992) (3rd Edn) Churchill
Livingstone, London 7: 142-161. 27.
Muretto
P. Lymphoid follicles in extranodal sites: an immunohistochemical study (1994)
Eur J Histochem. 38: 219-228. 28.
Muretto
P. An immunohistochemical study on foetuses and newborns lymph nodes with
emphasis on follicular dendritic reticulum cells (1995) Eur J Histochem. 39:
301-308. 29.
Muretto
P. Immunohistochemical study of tonsils from newborn infants with emphasis on
follicular dendritic reticulum cells (1998) Eur J Histochem (1998) 42: 189-195.
30.
Bertini
M. "Endothelial Protector Drugs" and Diabetes: Is there a Role for these
Drugs? (2015) J Obesity and Diabetics 1: 1-3 *Corresponding
author Diabetes Immunodeficiency, Lymph-nodes
microangiopathy, Lymphodepletion, Follicular dendritic reticulum cells.Diffuse Lymph-Nodes Microangiopathy as Concurrent Cause of Immunodeficiency in Long-Term Insulin-Dependent Diabetic Patients
Pietro Muretto
Abstract
Immune abnormalities
have been demonstrated in vitro models in genetic (type1) autoimmune (type 2) and
metabolic (type 1 and type 2) Insulin-Dependent Diabetes Mellitus (IDDM).
However, the precise reason for increased susceptibility to frequent and protracted
infections in diabetic patients is still unclear, despite a multitude of in
vitro studies which have focused on the metabolic and functional modifications
of immune cells Diabetes microangiopathy, which is a peculiar alteration of the
disease, has been extensively described in the retina, renal glomeruli, skin,
skeletal muscles, peripheral nerves, and other organs but not in lymph nodes.
We report our histological and immunohistochemical observations in lymph-nodes
removed in multiple sites during autopsy from four patients with long-term
IDDM, severe lymphocytpenia and several infectious diseases during their life. The peculiar
microangiopathic modifications made by presence of hyaline substance thickening
basal membrane of thin vessels and capillaries appear concurrent with
lymphodepletion of B and T cells dependent areas of lymph-nodes and with
jointed marked reduction of Follicular Dendritic Reticulum Cells (FDRC). Indeed
microangiopathy further compromise the traffic and diapedesis of T and B lymphocytes
may prevent the transformation of endothelial cells into FDRC with severe
immune failure of lymphoid follicles. The
histological and immunohistochemical data in this study could provide
additional insights into the complex problem of the immunodeficiency in
diabetic patients.
Full-Text
Introduction
Studies about modifications of lymph-nodes in diabetes patients have not been
found in literature even if these diffuse small organs of the human body have
specifically defensive functions against infection diseases. It is well known that lymph-nodes
are the prevalent site of lymphocytes production (B or T dependent) by which
they represent one of the best regulating factors in immunity, but further it
is necessary take present that their stromal structure if made of a very rich network
of capillaries and small vessels, which can easily highlighted by
immunohistochemistry with surprising microscopic views, explaining in some way
the microangiopathy localization in lymph-nodes.Patients and Methods
Results
Discussion
More
recent studies and reports focused on pathophysiology of infections associated
with diabetes mellitus refer negative effects on lymphocyte T and neutrophil functions
with increased apoptosis and lower secretion of inflammatory cytokines while
increased virulence of infectious microorganisms appears related to hyperglycemia
which further produce apoptosis reduces polymorphonuclear leucocyte transmigration
through the endothelium [2]. Regarding the lymphocytes some studies had
demonstrated that when the glycated hemoglobin (HBA1c) is less of 8% the proliferative function of CD4T
lymphocytes and their response to antigen is not impaired. others publications related to large population-based
observational studies have reported strong associations between higher HbA1c
and infection risks for both type 1 and type 2 diabetes [9-16].
Lymph nodes have not previously been the object of attention or study in
diabetes. The results of our histological and immunohistochemical observations
suggest the possibility of another mechanism potentially contributing to
impaired immunity in patients with diabetes. The lymph nodes removed at autopsy
on four patients with long-term IDDM have shown alteration of the normal
architecture and peculiar changes of vessels with capillary BMT.
lmmunohistochemistry confirmed the low level
of the T and B cells and further demonstrated a very low number of dendritic
reticulum cells. Between DRCs only few mature B lymphocytes and some scattered
small cleaved cells were observed (Figure 4).References
Pietro Muretto, Department of Surgical
Pathology, Pathologist and Hematologist, Ospedale San Salvatore Pesaro, ltaly, E-mail:
pietro.muretto@virgilio.it
Citation
Muretto P. Diffuse lymph-nodes microangiopathy as concurrent
cause of immunodeficiency in long-term insulin-dependent diabetic patients
(2019) J Obesity and Diabetes 3: 25-29 Keywords