Research Article :
Nereida Spahia,
Merita Rroji, Myftar Barbullushi and Mauro Sasdelli The Focal Segmental Glomerulosclerosis (FSGS) is
one of the most frequent glomerular nephropathies affecting both children and
adults. The aim of this study is the evaluation of the effects of Mycophenolate
Mofetil (MMF) in Nephrotic Syndrome (NS) with biopsy proven Focal Segmental
Glomerulosclerosis (FSGS) resistant to other therapies. We treated 20 patients,
of which 12 males, with a median age of 39 years (ranging between 18 and 62
years), with Nephrotic Syndrome, all being resistant to or relapsing on steroid
and immunosuppressive therapy. They were treated with MMF (1-2 g/day) and
Methylprednisolone 0.5 mg/kg at alternate days for an average period of ten
months (ranging between 3 and 13 months). Two patients discontinued treatment
after three and five months respectively, for gastric intolerance. Another
patient discontinued MMF after six months due to deterioration of kidney
function. No significant differences were observed between pretreatment values
and at the end of the treatment for plasma creatinine, Glomerular Filtration
Rate (GFR), while the excretion rate of urinary proteins was significantly
reduced from 7.68 ± 3.54 to 3.20 ± 2.92 g/day, (p<0.001). After MMF we
observed a complete remission in two patients (10%), an incomplete remission in
three patients (15%), a partial remission in six patients (30%), no response in
eight patients (40%) and a worsening of kidney function in one patient (5%). It
was concluded that in resistant Nephrotic Syndrome by FSGS, MMF can favor
stable remission, preserving renal function and hence being considered as an
alternative therapy to calcineurin inhibitors, but with lower toxicity. The
Focal Segmental Glomerulosclerosis (FSGS) is one of the most frequent
glomerular nephropathies affecting both children and adults. It may be
idiopathic or secondary to such causes known as the reduced nephron mass,
obesity, viral infection or drugs and toxins. The morphological/histological
pattern recognized on kidney biopsy is characterized by sclerotic (fibrotic)
lesions in glomeruli that are focal (less than 50% of all glomeruli affected on
light microscopy) and segmental (less than 50% of the glomerular tuft
affected). This pathological pattern has been further classified by the
Columbia group according to specific pathological light microscopic findings
(tip lesion, cellular, collapsing, perihilar and not otherwise specified).
Podocyte injury is the earliest morphological feature of FSGS, which has led to
the current paradigm that classic FSGS is primarily a podocyte disorder, at
least initially. The causes of podocyte damage can either be genetic or related
to circulating permeability factors. The prognosis of FSGS is predicted by the
severity and persistence of proteinuria, with 60% of patients with persistent
nephrotic-range proteinuria progressing to end-stage renal disease within 5-10
years. Achievement of a remission, whether complete or partial, is associated
with a good outcome [1-6]. At
present, corticosteroids are the standard first-line approach in patients with
idiopathic FSGS. Cytotoxic agents and cyclosporin A constitute a good
therapeutic option for steroid-dependent patients or frequent relapsers. During
the last years, the use of Mycophenolate Mofetil (MMF) has been proposed in the
Nephrotic Syndrome by FSGS together with steroids with varying results, but
with the advantage of a lower toxicity compared with other immunosuppressants. The
aim of this work is to report our experience with the therapy of MMF in a group
of idiopathic FSGS with Nephrotic Syndrome who have been previously treated
with steroids and immunosuppressive and who had not responded to treatment
showing a resistant or relapsing nephrotic syndrome [7-10]. There
were examined twenty patients with a histological diagnosis of Focal and
Segmental Glomerulosclerosis having a resistant or relapsing nephrotic
syndrome. The nephrotic syndrome was defined by a proteinuria>3 g/day, hypoalbuminuria<3g/dl
and oedema. The median age was 39 years ranging between 18 and 62 years old, in
which 12 were males? All patients had a nephrotic syndrome at onset and were
treated with various therapies and through different periods and dosages: six
patients with steroids alone (two with bolus of 500 mg x 3 days and then
steroids per os, four with steroids per os), eight patients with steroids +
cyclophosphamide, five patients with steroids + cyclosporine and one patient
steroids + azathioprine. All patients were examined on an outpatient basis
generally every 3 months with a clinical examination and control of the main tests
(plasma creatinine, 24-h urinary protein excretion rate, hemochrome, plasma
glucose, serum total protein, cholesterol and transaminase). The Glomerular
Filtrate (GFR) was calculated using the CKD-EPI formula. In
addition, all patients were recommended to follow a low-salt diet and used
other medications such as antihypertensives, statins, calcium supplements and
vitamin D3. All patients (after the prolonged therapeutic regimes previously
reported), which still presented the clinical and laboratory picture of
nephrotic syndrome where put on a regime with Mycophenolate (Cell Cept) 1 g/day
for a month and then, if well tolerated, the dose was increased to 2 g/day with
a first step at 6 months. If the Nephrotic Syndrome was in complete remission
MMF was suspended. Otherwise, it was continued for up to 12 months with
follow-up of the patients. In two cases with a creatinine level greater than 2
mg/dl and a glomerular filtrate less than 50 ml/min it was used a dose of
Mycophenolate of 1g/day. In
all patients, therapy with Methylprednisolone 0.5 mg/kg every other day,
Ramipril, Calcium and vitamin D3 was used. The clinical response was definied
as a Complete Remission (CR) if the rate of urinary protein excretion was
<0.3 g/day, an Incomplete Remission (IR) if the rate of urinary protein
excretion was between 0.3 and 1 g/day, Partial Remission (PR) if the rate of
urinary protein excretion was between 1 and 3 g/day, No Remission (NR) if the
urinary protein excretion remained >3 g/day, worsening (W) if there was an
increase in plasma creatinine of at least 50 % over the baseline value.The
results were expressed as means ± SD. Student’s t test was used for statistical
comparison of the means. Statistical
Analysis The
statistical analysis was performed with SPSS, (Statistical Package for Social
Sciences Inc., Chicago, IL, USA), version 19.0. Results were expressed as mean
± SD. Data were compared between groups by t test Patient
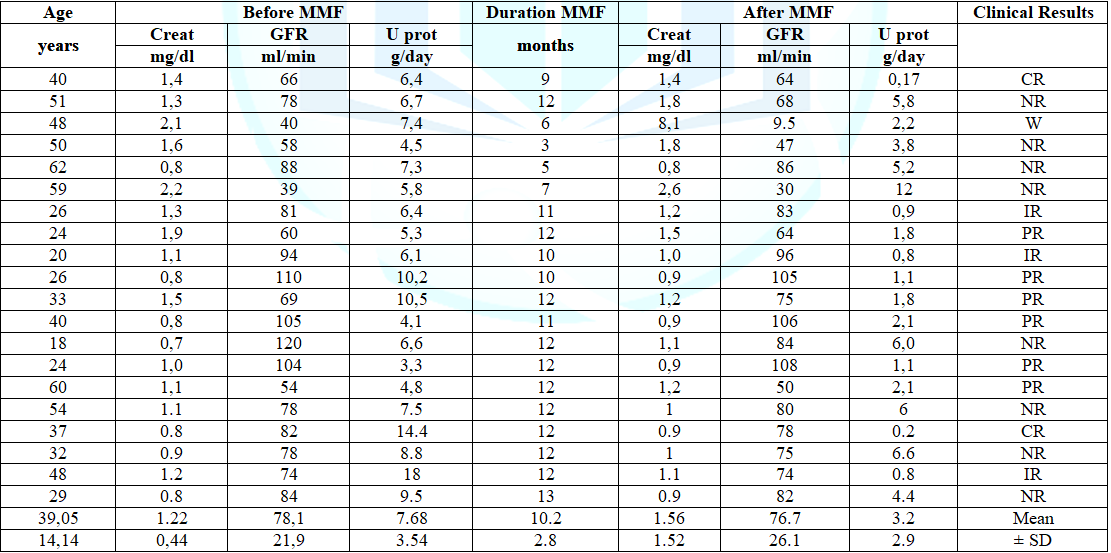
data before and after treatment with MMF and duration of therapy are presented
in table 1. Before starting MMF, the
median plasma cretonne was 1.22 ± 0.44 mg/dl with an average Glomerular
Filtrate Rate (GFR) of 78.1 ± 21.9 ml/min. In two patients the values of
creatinine were higher than 2 mg/dl (2.1 and 2.2 mg/dl) and GFR was <50
ml/min (40 and 39 ml/min). The 24 hours urinary protein excretion was 7.68 ±
3.54 g/day and the serum total proteins was 5.03 ± 0.38 g/dl. Eight patients
had hypertension treated with ACE-inhibitors or ARB. Two patients discontinued
treatment after 3 and 5 months respectively, for gastric intolerance. Table 1: Clinical data of the patients before and after MMF therapy The
initial treatment of primary FSGS usually involves corticosteroids. In
observational and uncontrolled trials, prolonged prednisone therapy (mean 9
months) in adults resulted in complete and partial remission rates in the
25-33% of patients. Improved outcome was suggested with long-term high-dose
pulse corticosteroid therapy in conjunction with cytotoxic agents compared with
historic controls. These studies suggest that long-term corticosteroid therapy
may improve the partial and complete remission rate in patients with FSGS and
resistance to a standard short course of corticosteroids. Randomized clinical
trials have shown the Cyclosporine (CSA) coupled with low-dose prednisone can
increase the rate of partial and complete remission, but this therapy suffers
the causing of high relapse rate following discontinuation of CSA and presents
the risk of frequent side effects, including nephrotoxicity. If the authors
agree to use steroids as initial treatment, there is a great variety in their
dosages and duration and then in the choice of drugs for the treatment of resistant
or recurrent forms [11-17]. In the last few years numerous authors have studied
the effects of MMF on FSGS [7-10]. The MMF is an immunosuppressant which acts
by inhibiting the purine synthesis by a selective, non-competitive and
reversible inhibition of inosine monophosphate dehydrogenase which is the
rate-limiting enzyme in the de novo biosynthesis of guanosine nucleotides. MMF
strongly inhibits both T-and B lymphocyte proliferation. Moreover,
MMF is also capable of inhibiting the proliferation of non-immune cells as
smooth muscle cells, renal tubular cells and mesangial cells and prevents the
appearance of Heymann Nephritis. In the animal studies, MMF reduces the
expression of nitric oxide synthase at the cortical level by decreasing
glomerulosclerosis and glomerular crescent formation, increasing the expression
of nephrine and podocin in diabetic rats, inhibits abnormal renal cell growth
by regulating cell cycle or apoptosis related genes, ameliorates renal lesions
in immune-mediated disease, but was also effective in non-immune-mediated renal
damage in the rat remnant-kidney model [18-23]. Initially it has been used in
the prevention of acute and chronic allograft rejection since the mid-1990s. MMF
showed beneficial effects in the treatment of calcineurin inhibitor toxicity
through reduction of immune - and non-immune-mediated renal damage. Nevertheless,
it is well tolerated and has proven to be a relatively safe drug causing only
minor bone marrow suppression. In addition, there is a growing body of evidence
pointing to therapeutic applications of MMF in the prevention of fibrosis. These
observations prompted several investigators to study the effects of MMF in
human renal diseases. It was noted that the MMF significantly reduced
proteinuria in minimal-change disease, especially in the steroid-resistant nephrotic
syndrome of children. In adults MMF has been used in various nephropathies: in
the lupus nephritis, where favorable results have been reported to maintain remission
of the disease, while in other nephropathies as the membranous nephropathy, the
IgA nephropathy, the membranoproliferative glomerulonephritis, ANCA-associated
vasculitis and FSGS, the results were very variable and even more controversial
[24-38]. In this work, there were examined twenty adult patients with a
biopsy-proven diagnosis of primitive Focal Segmental Glomelosclerosis who had a
Nephrotic Syndrome being treated with various therapies whithout positive
outcome. Regardless
of therapeutical regime applied, a clinical and laboratory picture of Nephrotic
Syndrome was still present in all of them. Afterwards, therapy with MMF
associated with Methylprednisolone was started and continued for 3-13 months
(on average 10.2 ± 2.8 months). At the end of MMF therapy we observed a
remission in eleven patients, two of which with complete remission and nine
with incomplete or partial remission. In eight patients, the Nephrotic Syndrome
remained unchanged, two of which suspended early therapy for side effects,
while in one case the therapy was suspended due to the worsening of renal
function. In total we have observed a remission in eleven patients (55%) and
this can be considered a good outcome, considering that these patients did not
respond to previous therapies. After treatment, the mean urinary protein
excretion significantly decreased compared with baseline, while plasma
creatinine and the glomerular filtrate did not show significant variations. In
our view this is an important result, as remission either complete or partial,
is the critical factor for predicting renal survival in nephrotic syndrome due
to primary FSGS with 5-year renal survival of about 90% [7]. In addition, the
results remain stable also at the end of follow up. Furthermore,
the MMF was generally well tolerated and only in 2 cases appeared to have
gastric intolerance and no alteration of hepatic enzymes were observed. One
patient had a deteroration of kidney function during the regime and started
hemodialysis treatment. The results of this study, despite the relatively low
number of patients, can have a clinical impact, considering the high remission
rate and the good side effect profile observed with MMF regime in the treatment
of Nephrotic Syndrome due to FSGS resistant to other drugs. These results
confirm what was reported by other authors [39,40]. Hence, we may conclude that
in the treatment of resistant Nephrotic Syndrome due to FSGS, MMF represents a
therapeutic alternative with favorable effects and most importantly with
reduced side effects, in comparison to Calcineurin inhibitors such as
Cyclosporine, which however has a high renal toxicity [41,37]. Our contribution
is limited by the lack of casistic and it is retrospective study, but the
results are encouraging and we believe that it deserves to be taken into
consideration and to be confirmed by further larger studies comparing the MMF
with other drugs recommended by the literature. Our
results give an additional confirmation for the benefits of MMF regime in FSGS.
Hence, we may conclude that in the treatment of resistant Nephrotic Syndrome
due to FSGS, MMF represents a therapeutic alternative with favorable effects
and most importantly with reduced side effects, in comparison to Calcineurin
inhibitors such as Cyclosporine, which however has a high renal toxicity. Ethical Approval
This study was in
accordance with the ethical standards of the institutional and/or national
research committee and with the 1964 Helsinki declaration and its later
amendments or comparable ethical standards. Mauro Sasdelli, Ex Chief of Nephrology, Department of St. Donato
Hospital, Arezzo, Italy, Email: maurolli@libero.it
Spahia N, Rroji M,
Barbullushi M and Sasdelli M. The mycophenolate mofetil therapy in
corticoresistent idiopathic focal segmental glomerulosclerosis (2020) J Obesity
and Diabetes 4: 1-4. Glomerulosclerosis, Idiopathic Focal Segment, Hypoalbuminuria.The Mycophenolate Mofetil Therapy in Corticoresistent Idiopathic Focal Segmental Glomerulosclerosis
Abstract
Full-Text
Introduction
Patients and
Methods
Results
Discussion
Conclusion
References
*Corresponding author
Citation
Keywords