Introduction
The
Focal Segmental Glomerulosclerosis (FSGS) is one of the most frequent
glomerular nephropathies affecting both children and adults. It may be
idiopathic or secondary to such causes known as the reduced nephron mass,
obesity, viral infection or drugs and toxins. The morphological/histological
pattern recognized on kidney biopsy is characterized by sclerotic (fibrotic)
lesions in glomeruli that are focal (less than 50% of all glomeruli affected on
light microscopy) and segmental (less than 50% of the glomerular tuft
affected). This pathological pattern has been further classified by the
Columbia group according to specific pathological light microscopic findings
(tip lesion, cellular, collapsing, perihilar and not otherwise specified).
Podocyte injury is the earliest morphological feature of FSGS, which has led to
the current paradigm that classic FSGS is primarily a podocyte disorder, at
least initially. The causes of podocyte damage can either be genetic or related
to circulating permeability factors. The prognosis of FSGS is predicted by the
severity and persistence of proteinuria, with 60% of patients with persistent
nephrotic-range proteinuria progressing to end-stage renal disease within 5-10
years. Achievement of a remission, whether complete or partial, is associated
with a good outcome [1-6].
At present, corticosteroids are the standard first-line approach in patients with idiopathic FSGS. Cytotoxic agents and cyclosporin A constitute a good therapeutic option for steroid-dependent patients or frequent relapsers. During the last years, the use of Mycophenolate Mofetil (MMF) has been proposed in the Nephrotic Syndrome by FSGS together with steroids with varying results, but with the advantage of a lower toxicity compared with other immunosuppressants. The aim of this work is to report our experience with the therapy of MMF in a group of idiopathic FSGS with Nephrotic Syndrome who have been previously treated with steroids and immunosuppressive and who had not responded to treatment showing a resistant or relapsing nephrotic syndrome [7-10].
Patients and Methods
There
were examined twenty patients with a histological diagnosis of Focal and
Segmental Glomerulosclerosis having a resistant or relapsing nephrotic
syndrome. The nephrotic syndrome was defined by a proteinuria>3 g/day, hypoalbuminuria<3g/dl
and oedema. The median age was 39 years ranging between 18 and 62 years old, in
which 12 were males? All patients had a nephrotic syndrome at onset and were
treated with various therapies and through different periods and dosages: six
patients with steroids alone (two with bolus of 500 mg x 3 days and then
steroids per os, four with steroids per os), eight patients with steroids +
cyclophosphamide, five patients with steroids + cyclosporine and one patient
steroids + azathioprine. All patients were examined on an outpatient basis
generally every 3 months with a clinical examination and control of the main tests
(plasma creatinine, 24-h urinary protein excretion rate, hemochrome, plasma
glucose, serum total protein, cholesterol and transaminase). The Glomerular
Filtrate (GFR) was calculated using the CKD-EPI formula.
In addition, all patients were recommended to follow a low-salt diet and used other medications such as antihypertensives, statins, calcium supplements and vitamin D3. All patients (after the prolonged therapeutic regimes previously reported), which still presented the clinical and laboratory picture of nephrotic syndrome where put on a regime with Mycophenolate (Cell Cept) 1 g/day for a month and then, if well tolerated, the dose was increased to 2 g/day with a first step at 6 months. If the Nephrotic Syndrome was in complete remission MMF was suspended. Otherwise, it was continued for up to 12 months with follow-up of the patients. In two cases with a creatinine level greater than 2 mg/dl and a glomerular filtrate less than 50 ml/min it was used a dose of Mycophenolate of 1g/day.
In all patients, therapy with Methylprednisolone 0.5 mg/kg every other day, Ramipril, Calcium and vitamin D3 was used. The clinical response was definied as a Complete Remission (CR) if the rate of urinary protein excretion was <0.3 g/day, an Incomplete Remission (IR) if the rate of urinary protein excretion was between 0.3 and 1 g/day, Partial Remission (PR) if the rate of urinary protein excretion was between 1 and 3 g/day, No Remission (NR) if the urinary protein excretion remained >3 g/day, worsening (W) if there was an increase in plasma creatinine of at least 50 % over the baseline value.The results were expressed as means ± SD. Student’s t test was used for statistical comparison of the means.
Statistical
Analysis
The statistical analysis was performed with SPSS, (Statistical Package for Social Sciences Inc., Chicago, IL, USA), version 19.0. Results were expressed as mean ± SD. Data were compared between groups by t test
Results
Patient
data before and after treatment with MMF and duration of therapy are presented
in table 1. Before starting MMF, the
median plasma cretonne was 1.22 ± 0.44 mg/dl with an average Glomerular
Filtrate Rate (GFR) of 78.1 ± 21.9 ml/min. In two patients the values of
creatinine were higher than 2 mg/dl (2.1 and 2.2 mg/dl) and GFR was <50
ml/min (40 and 39 ml/min). The 24 hours urinary protein excretion was 7.68 ±
3.54 g/day and the serum total proteins was 5.03 ± 0.38 g/dl. Eight patients
had hypertension treated with ACE-inhibitors or ARB. Two patients discontinued
treatment after 3 and 5 months respectively, for gastric intolerance.
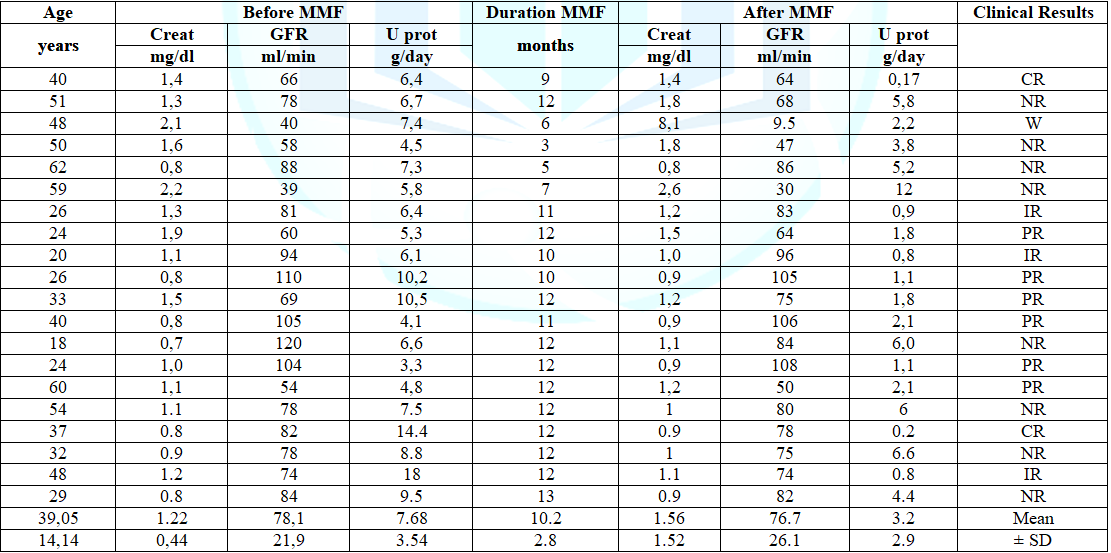
Table 1: Clinical data of the patients before and after MMF therapy
Discussion
The
initial treatment of primary FSGS usually involves corticosteroids. In
observational and uncontrolled trials, prolonged prednisone therapy (mean 9
months) in adults resulted in complete and partial remission rates in the
25-33% of patients. Improved outcome was suggested with long-term high-dose
pulse corticosteroid therapy in conjunction with cytotoxic agents compared with
historic controls. These studies suggest that long-term corticosteroid therapy
may improve the partial and complete remission rate in patients with FSGS and
resistance to a standard short course of corticosteroids. Randomized clinical
trials have shown the Cyclosporine (CSA) coupled with low-dose prednisone can
increase the rate of partial and complete remission, but this therapy suffers
the causing of high relapse rate following discontinuation of CSA and presents
the risk of frequent side effects, including nephrotoxicity. If the authors
agree to use steroids as initial treatment, there is a great variety in their
dosages and duration and then in the choice of drugs for the treatment of resistant
or recurrent forms [11-17]. In the last few years numerous authors have studied
the effects of MMF on FSGS [7-10]. The MMF is an immunosuppressant which acts
by inhibiting the purine synthesis by a selective, non-competitive and
reversible inhibition of inosine monophosphate dehydrogenase which is the
rate-limiting enzyme in the de novo biosynthesis of guanosine nucleotides. MMF
strongly inhibits both T-and B lymphocyte proliferation.
Moreover,
MMF is also capable of inhibiting the proliferation of non-immune cells as
smooth muscle cells, renal tubular cells and mesangial cells and prevents the
appearance of Heymann Nephritis. In the animal studies, MMF reduces the
expression of nitric oxide synthase at the cortical level by decreasing
glomerulosclerosis and glomerular crescent formation, increasing the expression
of nephrine and podocin in diabetic rats, inhibits abnormal renal cell growth
by regulating cell cycle or apoptosis related genes, ameliorates renal lesions
in immune-mediated disease, but was also effective in non-immune-mediated renal
damage in the rat remnant-kidney model [18-23]. Initially it has been used in
the prevention of acute and chronic allograft rejection since the mid-1990s. MMF
showed beneficial effects in the treatment of calcineurin inhibitor toxicity
through reduction of immune - and non-immune-mediated renal damage.
Nevertheless,
it is well tolerated and has proven to be a relatively safe drug causing only
minor bone marrow suppression. In addition, there is a growing body of evidence
pointing to therapeutic applications of MMF in the prevention of fibrosis. These
observations prompted several investigators to study the effects of MMF in
human renal diseases. It was noted that the MMF significantly reduced
proteinuria in minimal-change disease, especially in the steroid-resistant nephrotic
syndrome of children. In adults MMF has been used in various nephropathies: in
the lupus nephritis, where favorable results have been reported to maintain remission
of the disease, while in other nephropathies as the membranous nephropathy, the
IgA nephropathy, the membranoproliferative glomerulonephritis, ANCA-associated
vasculitis and FSGS, the results were very variable and even more controversial
[24-38]. In this work, there were examined twenty adult patients with a
biopsy-proven diagnosis of primitive Focal Segmental Glomelosclerosis who had a
Nephrotic Syndrome being treated with various therapies whithout positive
outcome.
Regardless
of therapeutical regime applied, a clinical and laboratory picture of Nephrotic
Syndrome was still present in all of them. Afterwards, therapy with MMF
associated with Methylprednisolone was started and continued for 3-13 months
(on average 10.2 ± 2.8 months). At the end of MMF therapy we observed a
remission in eleven patients, two of which with complete remission and nine
with incomplete or partial remission. In eight patients, the Nephrotic Syndrome
remained unchanged, two of which suspended early therapy for side effects,
while in one case the therapy was suspended due to the worsening of renal
function. In total we have observed a remission in eleven patients (55%) and
this can be considered a good outcome, considering that these patients did not
respond to previous therapies. After treatment, the mean urinary protein
excretion significantly decreased compared with baseline, while plasma
creatinine and the glomerular filtrate did not show significant variations. In
our view this is an important result, as remission either complete or partial,
is the critical factor for predicting renal survival in nephrotic syndrome due
to primary FSGS with 5-year renal survival of about 90% [7]. In addition, the
results remain stable also at the end of follow up.
Furthermore, the MMF was generally well tolerated and only in 2 cases appeared to have gastric intolerance and no alteration of hepatic enzymes were observed. One patient had a deteroration of kidney function during the regime and started hemodialysis treatment. The results of this study, despite the relatively low number of patients, can have a clinical impact, considering the high remission rate and the good side effect profile observed with MMF regime in the treatment of Nephrotic Syndrome due to FSGS resistant to other drugs. These results confirm what was reported by other authors [39,40]. Hence, we may conclude that in the treatment of resistant Nephrotic Syndrome due to FSGS, MMF represents a therapeutic alternative with favorable effects and most importantly with reduced side effects, in comparison to Calcineurin inhibitors such as Cyclosporine, which however has a high renal toxicity [41,37]. Our contribution is limited by the lack of casistic and it is retrospective study, but the results are encouraging and we believe that it deserves to be taken into consideration and to be confirmed by further larger studies comparing the MMF with other drugs recommended by the literature.
Conclusion
Our results give an additional confirmation for the benefits of MMF regime in FSGS. Hence, we may conclude that in the treatment of resistant Nephrotic Syndrome due to FSGS, MMF represents a therapeutic alternative with favorable effects and most importantly with reduced side effects, in comparison to Calcineurin inhibitors such as Cyclosporine, which however has a high renal toxicity.
Ethical Approval
This study was in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Jefferson AJ and Shankland SJ. The pathogenesis of focal segmental glomerulosclerosis (2014) Adv Chronic Kidney Dis 21: 408-416. https://doi.org/10.1053/j.ackd.2014.05.009
- D'Agati VD, Fogo AB, Bruijn JA and Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal (2014) Am J Kidney Dis 43: 368-382. https://doi.org/10.1053/j.ajkd.2003.10.024
- Parsa A, WH Linda Kao, Xie D, Brad C Astor, Man Li, et al. APOL1 risk variants, race, and progression of chronic kidney disease (2013) N Engl J Med 369: 2183-2196. https://doi.org/10.1056/nejmoa1310345
- Reiser J, Cynthia C Nast, and Nada Alachkar. Permeability Factors in Focal and Segmental Glomerulosclerosis (2014) Adv Chronic Kidney Dis 21: 417-421. https://doi.org/10.1053/j.ackd.2014.05.010
- Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC, et al. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission (2005) J Am Soc Nephrol 16:1051-1068. https://doi.org/10.1681/asn.2004070593
- Deegens JK and Wetzels JF. Immunosuppressive treatment of focal segmental glomerulosclerosis: lessons from a randomized controlled trial (2011) Kidney Int 80: 798-801. https://doi.org/10.1038/ki.2011.191
- Cattran DC, Wang MM, Appel G, Matalon A and Briggs W. Mycophenolate mofetil in the treatment of focal segmental glomerulosclerosis (2004) Clin Nephrol 62: 405-411. https://doi.org/10.5414/cnp62405
- Deegens JK, Steenbergen EJ and Wetzels JF. Review on diagnosis and treatment of focal segmental glomerulosclerosis (2008) Neth J Med 66: 3-12.
- Braun N, Schmutzler F, Lange C, Perna A, Remuzzi G, et al. Immunosuppressive treatment for focal segmental glomerulosclerosis in adults (2008) Cocrhane Databese Syst Rev 16: CD003233. https://doi.org/10.1002/14651858.cd003233.pub2
- Ponticelli C and Graziani G. Current and emerging treatments for idiopathic focal and segmental glomerulosclerosis in adults (2013) Expert Rev Clin Immunol 9: 251-261. https://doi.org/10.1586/eci.12.109
- Tune BM and Mendoza SA. Treatment of the idiopathic nephrotic syndrome: regimens and outcomes in children and adults (1997) J Am Soc Nephrol 8: 824-832.
- Alexopoulos E, Stangou M, Papagianni A, Pantzaki A and Papadimitriou M. Factors influencing the course and the response to treatment in primary focal segmental glomerulosclerosis (2000) Nephrol Dial Transplan 15: 1348-1356. https://doi.org/10.1093/ndt/15.9.1348
- Moranne O, Watier L, Rossert J and Stengel B. Primary glomerulonephritis: an update on renal survival and determinants of progression (2008) QJM 101: 215-224. https://doi.org/10.1093/qjmed/hcm142
- Passerini P, Scolari F, Frasca GM, Leoni A, Venturelli C, et al. Controversial issues in the Giornale Italiano di Nefrologia: how to treat patients with focal segmental glomerular sclerosis (2009) G Ital Nefrol 26: 563-576.
- Cattran, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group (1999) Kidney Int 56: 2220-2226. https://doi.org/10.1046/j.1523-1755.1999.00778.x
- Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults (2011) Kidney Int 80: 868-878. https://doi.org/10.1038/ki.2011.195
- Fernandez-Juarez G, Villacorta J and Ortiz A. Therapeutic variability in adult minimal change disease and focal segmental glomerulosclerosis (2016) Clin Kidney J 9: 381-386.
- Morath C and Zeier M. Review of the antiproliferative properties of mycophenolate mofetil in non-immune cells (2003) Int J Clin Pharmacol Ther 41: 465-469. https://doi.org/10.5414/cpp41465
- Penny MJ, Boyd RA and Hall BM. Mycophenolate mofetil prevents the induction of active Heymann nephritis: association with Th2 cytokine inhibition (1998) J Am Soc Nephrol 12: 2272-2282.
- Wu Y, Dong J, Yuan L, Liang C, Ren K, et al. Shen Nephrin and podocin loss is prevented by mycophenolate mofetil in early experimental diabetic nephropathy (2008) J Citokine 244: 85-91. https://doi.org/10.1016/j.cyto.2008.06.015
- Lv W, ingqiu Lou and Jianping Wang. Mycophenolate mofetil inhibits hypertrophy and apoptosis of podocyte in vivo and in vitro (2015) Int J Clin Exp Med 8: 19781-19790.
- Ziswiler R, Steinmann-Niggli K, Kappeler A, Daniel C and Marti HP. Mycophenolic acid: a new approach to the therapy of experimental mesangial proliferative glomerulonephritis (1998) J Am Soc Nephrol 11: 2055-2066.
- Takeda S, Masafumi Takahashi, Yoshikazu Sado, Koichi Takeuchi, Yoji Hakamata, et al. Prevention of glomerular crescent formation in glomerulonephritis by mycophenolate mofetil in rats (2004) Nephrol Dial Transplant 19: 2228-2236. https://doi.org/10.1093/ndt/gfh30
- Hauser IA and Sterzel RB. Mycophenolate mofetil: therapeutic applications in kidney transplantation and immune-mediated renal disease (1999) Curr Opin Nephrol Hypertens 8: 1-6. https://doi.org/10.1097/00041552-199901000-00001
- Yang CW, Hee Jong Ahn, Wan Young Kim, Can Li, Hyung Wook Kim, et al. Cyclosporine withdrawal and mycophenolate mofetil treatment effects on the progression of chronic cyclosporine nephrotoxicity (2002) Kidney Int 62: 20-30. https://doi.org/10.1046/j.1523-1755.2002.00400.x
- Morath C, Schwenger V, Beimler J, Mehrabi A, Schmidt J, et al. Antifibrotic actions of mycophenolic acid (2005) Clin Transplant 17: 25-29. https://doi.org/10.1111/j.1399-0012.2006.00597.x
- Siu YP, Tong MK, Leung Ka, Kwan TH, Au TC. The use of enteric-coated mycophenolate sodium in the treatment of relapsing and steroid-dependent minimal change disease (2008) J Nephrol 21: 127-131.
- Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis (2011) N Engl J Med 365: 1886-1895. https://doi.org/10.1056/nejmoa1014460
- Palmer SC, Tunnicliffe DJ, Singh-Grewal D, Mavridis D, Tonelli M, et al. Induction and Maintenance Immunosuppression Treatment of Proliferative Lupus Nephritis: A Network Meta-analysis of Randomized Trials (2017) Am J Kidney Dis 70: 324-336. https://doi.org/10.1053/j.ajkd.2016.12.008
- Branten AJ, du Buf-Vereijken PW, Vervloet M and Wetzels JF. Mycophenolate mofetil in idiopathic membranous nephropathy: a clinical trial with comparison to a historic control group treated with cyclophosphamide (2007) Am J Kidney Dis 50: 248-256. https://doi.org/10.1053/j.ajkd.2007.05.015
- Senthil Nayagam L, Ganguli A, Rathi M, Kohli HS, Gupta KL, et al. Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: a pilot study (2008) Nephrol Dial Transplant 13: 1926-1930. https://doi.org/10.1093/ndt/gfm538
- Du B, Jia Y, Zhou W, Min X, Miao L, et al. Efficacy and safety of mycophenolate mofetil in patients with IgA nephropathy: an update meta-analysis (2017) BMC Nephrol 18: 245. https://doi.org/10.1186/s12882-017-0647-x
- Jones G, Juszczak M, Kingdon E, Harber M, Sweny P, et al. Treatment of idiopathic membranoproliferative glomerulonephritis with mycophenolate mofetil and steroids (2004) Nephro Dial Transplant 19: 3160-3164. https://doi.org/10.1093/ndt/gfh526
- Yuan M, Zou J, Zhang X, Liu H, Teng J, et al: Combination therapy with mycophenolate mofetil and prednisone in steroid-resistant idiopathic membranoproliferative glomerulonephritis (2010) Clin Nephrol 73: 354-359.https://doi.org/10.5414/cnp73354
- Yates M, Watts R, Bajema I, Cid M, Crestani B, et al. Validation of the EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis by disease content experts (2017) RMD Open 3: e000449. https://doi.org/10.1136/rmdopen-2017-000449
- Jeroen KJ and Deegens. Immunosuppressive treatment of focal segmental glomerulosclerosis: lessons from a randomized controlled trial (2011) Kidney Int 80: 798-801. https://doi.org/10.1038/ki.2011.191
- Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults (2011) Kidney Int 80: 868-878. https://doi.org/10.1038/ki.2011.195
- Beer A, Mayer G and Kronbichler A. Treatment Strategies of Adult Primary Focal Segmental Glomerulosclerosis: A Systematic Review Focusing on the Last Two Decades (2016) Biomed Res Int 216: 4192578. https://doi.org/10.1155/2016/4192578
- Li Z, Duan C, He J, Wu T, Xun M, et al: Mycophenolate mofetil therapy for children with steroid-resistant nephrotic syndrome (2010) Pediatr Nephrol 25: 883-888. https://doi.org/10.1007/s00467-009-1375-7
- Bagchi S, Agarwal S, Kalaivani M, Bhowmik D, Singh G, et al. FSGS in Nephrotic Adults: Clinical Profile, Response to Immunosuppression and Outcome (2016) Nephron 132: 81-85. https://doi.org/10.1159/00044299
- Ponticelli C, Rizzoni G, Edefonti A, Altieri P, Rivolta E, et al. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome (1993) Kidney Int 43: 1377-1384. https://doi.org/10.1038/ki.1993.194
*Corresponding author
Mauro Sasdelli, Ex Chief of Nephrology, Department of St. Donato
Hospital, Arezzo, Italy, Email: maurolli@libero.it
Citation
Spahia N, Rroji M, Barbullushi M and Sasdelli M. The mycophenolate mofetil therapy in corticoresistent idiopathic focal segmental glomerulosclerosis (2020) J Obesity and Diabetes 4: 1-4.
Keywords
Glomerulosclerosis, Idiopathic Focal Segment, Hypoalbuminuria.


 PDF
PDF