Research Article :
Tofail Ahmed, Hajera Mahtab,
Tania Tofail, AHG Morshed, Fatema B Rahman and Shahidul A Khan Introduction: Low Dose Overnight Dexamethasone Supression Test
(LODST) is a diagnostic tool for spontaneous Cushing’s Syndrome (CS). A LODST
negative excludes CS. But there are 2 exceptions - testing during silent period
of Cyclic Cushing’s Disease (CD) or a false negativity by one mg dexamethasone
in mild CD. Method: We analyzed age
and sex data of 154 LOSDT to see their risk association for CS.
Result: The detection rate of CS by LOSDT is 26% and with Cortisol
(211.27 to 373.69 nmol/L as 95% CI). Among the cases, 29.2% are pediatric and
70.8% are female. CS group do not differ from rest in sex and age group
distributions (sig.> 136) but CS is older group with a mean difference of
2.46 - 13.31 years (sig 005). Logistic equation documented CS is a different population
(sig 000) and which is influence by their age (sig 021) but not by sex or age
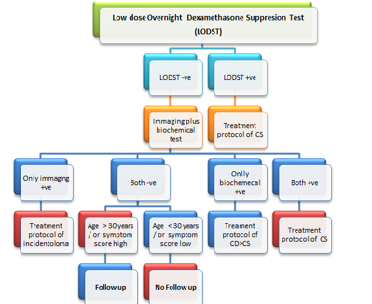
group (sig > 743). Therefore, age is an independent risk factor for CS. Conclusion: We opine to use LODST as
the first tool for CS. And LODST negative cases to be evaluated by newer
imaging and biochemical tests. Only in imaging positive are to be managed as
per guideline(s) for incidentaloma. Both negative cases are to be enrolled in
follow up if age > 30 years or symptoms score suggest CD and rest are to be
excluded. Cumulative diagnostic and outcome data will then may be used to
formulate cost-effective management policy for CS. Spontaneous
Cushing’s Syndrome (CS) is relatively rare disorder. The estimated incidence of
CS is 0.2-5 per million people per year and 66-70% of patients is due to Cushing’s
Disease (CD). LODST is a tool to documentation of spontaneous CS. It is
indicated for clinically suspected CS and to evaluate functional status of
adrenal incidentaloma. Suppressed cortisol value excludes spontaneous CS. But
there are at least two exceptions. One, when test is done during the silent
period in episodic variant of CD and other one is when 1 mg dexamethasone is
high dose to produce false negative in early or mild cases. There is a
continued search of more sensitive biochemical and imaging tests with a trend
of involving multidiscipline for more efficiently CS management. In this
context, we analyzed our laboratory data of LODST to reassess its’ current role
[1-22]. To
assess the utility of LODST we analyzed the available data (cortisol, age and
sex) of all 154 LODST done from July 2017 to December
2019 in Endocrinology Laboratory of Bangladesh Institute for Research and
Rehabilitation in Diabetes, Endocrine and Metabolic Disorders (BIRDEM). Twenty
six percent of cases (n=40) were positive for CS. We analyzed the data between
2 groups in search of utility of this tool. Test procedure and
interpolation of LODST: Dexamethasone 1 mg is administered orally between 11
PM and midnight. Blood is drawn for serum cortisol levels in the next morning
between 8 and 9 AM. A serum cortisol level < 50 nmol/L is considered as
suppressed meaning exclusion of spontaneous hypercortisolemia. Assay method: We used two
machines for Cortisol assay 1. ARCHITECT Cortisol assay which is a delayed
one-step immunoassay using CMIA technology (Chemiflex) by Abbott i2000 machine.
Its detection limit of serum sample is (41.385-14484.75 nmol/L) and 2. AVIDA
Centura XP assay which is a competitive immunoassay using CMIA technology by
SIEMENS immunosystem. Its detection limit of serum sample is (0.20 -2069
nmol/L). Data: Variable included
are cortisol, age, sex and Age group. Population is divided into suppressed and
no suppressed and groups comparison and logistic analysis to find risk
factor(s) for non- suppression. Comparison between groups for continuous
variable (cortisol) was done by independent sample t-test and for logical
variable (sex and age group) by chi square in cross table. We used IBM SPSS
statistics 20 for this purpose. Of
154 cases, 45(29.2%) were in pediatric age group (age <19 years) and rest
109 adult; and 109 (70.8%) were female & rest 45 male. By LODST complete
suppression (Cortisol < 50 nmol/L) occurred in 114 (74%) cases. So detection
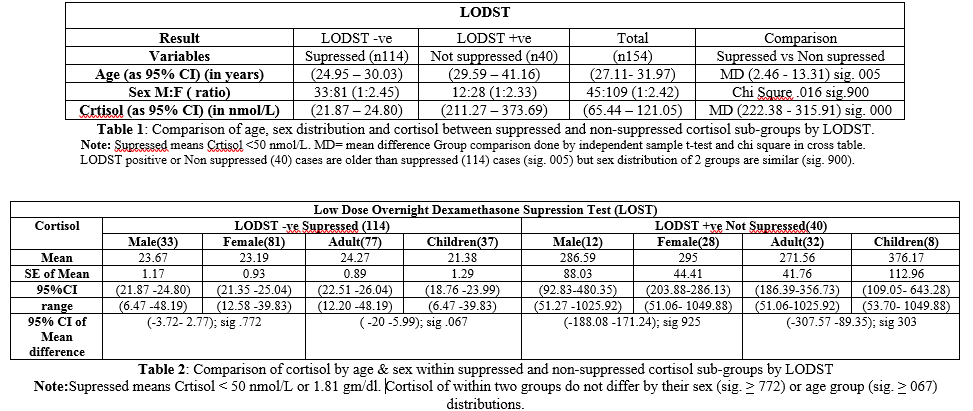
rate of ODST is 26% in this study population. Descriptive data Cortisol
of Supressed population expressed as mean + SE
of mean; 95% CI & (range) innmol/L. For Total (n=114): 23.34 + 0.74;
(21.87-24.80) and (6.47-48.19). ·
For
male (n=33): 23.67 + 1.17; (21.28
-26.06) and (12.58 -39.83). ·
And
for female (n=81): 23.19 + 0.93;
(21.35-25.04) and (6.47- 48.19). ·
For
Adult (n=77): 24.27 + 0.89;
(22.51-26.04) and (12.20-48.19). ·
And
Children (n=37): 21.38 + 01.29;
(18.76 – 23.99) and (6.47- 39.83) (Tables
1 and 2). Cortisol
of Not Suppressed population expressed as mean + SE of mean; 95%CI and (range)
innmol/L. For
Total (n=40): 292.48 + 40.15;
(211.27-373.69) and (51.06- 1049.88). ·
For
male (n=12): 286.59 + 88.03;
(92.83-480.35) and (51.27- 1025.92). ·
And
for female (n =28): 295.00 + 44.41;
(203.88 -286.13) and (51.06-1049.88). ·
For
Adult (n=32): 271.56 + 41.76;
(186.39- 356.73) and (51.06 -1025.92). ·
And
Children (n=8): 376.17 + 112.96;
(109.05 -643.28) and (53.70 -1049.88) (Tables
1 and 2). In
population of Suppressed cortisol (n=114) Mean Difference (MD) of cortisol
between male (n=33) and female (n=81): as mean + SE of mean and (95%CI) .48+
1.62(-3.72 – 2.77); sig. 772. And between adult (n=77) and children (n=37)
2.89+ 1.56(-.20 – 5.99) sig. 067 (Table
2). In population of Non Suppressed cortisol (n= 40) MD of cortisol between
male (n12) and female (n28) as mean +
SE of mean and
(95%CI) 8.42 + 88.75 (-188.08 –
171.24); sig. 925 and between adult (n32) and children (n8) 104.61 + 100.26(-307.57- 89.35) sig. 303. See
table 2.Therefore, there is no difference in cortisol level (sig. < .067) by
age group and sex in either of the population. See table 2.MD of age between
population of Supressed cortisol (n114) and Not Supressed cortisol (n40) as
mean + SE of mean and (95%CI) 7.88
+ 2.74 (2.46 -13.31), sig.005 (Table 1). Binary
logistic regression equation with age, sex & age group distribution as
covariate document that the suppressed and non- suppressed populations are
different (sig.000) and such deference is influence by their age (sig..021) but
not by sex (sig.821) or age group (sig.743) (Figures 1 and 2). Figure 1: Cortisol levels
during LODST. Like
other aspects of endocrinology, the spectrum of CS is constantly under
evolution. And it is in fact due to multidisciplinary involvement in its
management. We observed there are already some changes of frequencies of its different
symptom. This shifted the Cushing’s appearance of CS to a minimum or early
feature of hypercortisolema. This is the result of increased early delectation
of adrenal incidentaloma and they are mostly asymptomatic cases if identified
as CS. Moreover, due to involvement of neurosurgery and imaging specialist more
and more CD cases are being detected and treated. Our study documented the
detection rate of Cushing Syndrome by LODST is 26% in a mixed population of
symptomatic and or asymptomatic (adrenal incidentaloma) for CS. Considering the
availability, detection rate and cost we opine the LODST should remain as the
initial screening tool of investigation CS has it has been supported by other
studied [23-27]. The
newer, more sophisticated and sensitive tools like free cortisol in urine or
saliva, lower dose suppression test(30); CRF stimulated inferior petrosal sinus
sampling etc. should be in practice in specialized units in negative ODST
cases. An imaging at pituitary is need for all LODST negative cases. CT
scanning of the adrenal gland and MRI of the pituitary gland are performed to
detect the presence of any adrenal or pituitary adenomas or incidentalomas.
Scintigraphy of the adrenal gland with iodocholesterol scan is now may be needed
(35). Petrosal sinus sampling and ACTH assay is necessary for cases of
Cushing's disease are less than 2 mm in size and difficult to detect using MRI
or CT imaging. Conventional
MRI (CMRI) are now being replaced by Dynamic Contrast-Enhanced MRI (DMRI) and Spoiled
Gradient–Recalled Acquisition (SPGR) which have the potentiality to increase
value of MRI for CD due to micro adenoma will increase in the days to come. When
imaging is positive but biochemical test is/are negative than we can follow a guideline
for incidentoloma. Recent trend of shifting symptomatic (Cushingoid) to
asymptomatic (minimum symptoms presentation of CS is supported by the raised
prevalence of CD. An involvement of neuroradiology and neurosurgery with
endocrinology has definite contribution in this challenging area of
endocrinology [28-36]. In
the present study we have documented age as an independent risk factor for CS
so we proposed when standard imaging and biochemical tools are negative but age
is > 30 years they need to be enrolled in follow-up protocols along with
cases clinically suspected to be in silent phase of episodic variant of CD.
Newer biochemical and imaging tests for CS/CD should be done in specialized center.
Analysis of diagnostic and outcome data together has potential to develop
simpler and cost-effective management policy for CS.
We opine to use LODST as the screening tool
for Cushing Syndrome because of its good detection rate and availability for
long time. Then all LODST negative cases should be subjected to dual diagnostic
tools - imaging (the pituitary and adrenal) plus at least one newer biochemical
test (UFC/ Salivary/ other). Negative cases for both the tools need to be
enrolled in follow up protocols if age > 30 years or if symptoms score
suggest episodic variant of CD and rest can be excluded from follow up.
Positive in imaging tool but negative in biochemical tool(s) should be managed
according to guideline (s) for incidentaloma (Table 3). Note:
Biochemical tools are urinary or salivary cortisol or others such as very low
dose dexamethasone suppression, Inferior petrosal sinus sampling combining CRF
stimulation etc. Imaging tools MRI of Pituitary (preferably newer version) or
CT of adrenals etc. Tofail Ahmed, Department of Endocrinology, BIRDEM, Diabetic Association of
Bangladesh, Bangladesh, Email:
tofail.ahmed@yahoo.com
Ahmed T, Mahtab H, Tofail T, Morshed AHG,
Rahman BR and Khan AS. Current status of low dose overnight dexamethasone
supression test (LODST) (2020) J Obesity and Diabetes 4: 5-8. Low dose Overnight Dexamethasone Suppression Test,
Cushing’s syndrome, Cushing’s disease, Cortisol and detection rate of CS. Current Status of Low Dose Overnight Dexamethasone Supression Test (LODST)
Abstract
Full-Text
Introduction
Method
Result
Comparison data
Logistic analysis
data
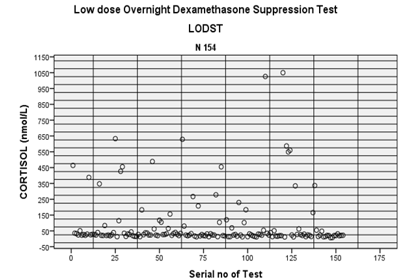
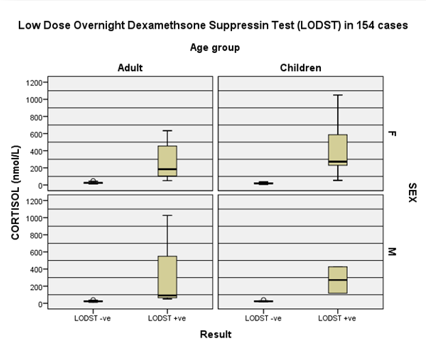
Figure 2: Comparison of sex
and age group data during LODST. Therefore, advancing age is an independent risk factor
for CS.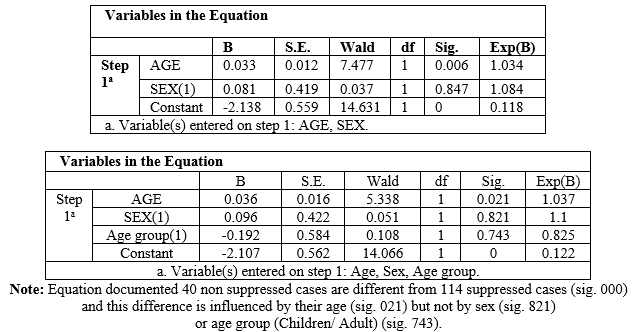
Discussion
References
*Corresponding author:
Citation:
Keywords
Abbreviations: LODST-Low
dose Overnight Dexamethasone Suppression Test, CS-Cushing’s
syndrome,
CD-Cushing’s
disease, DMRI-Dynamic
Contrast-Enhanced MRI, BIRDEM- Bangladesh Institute for Research and Rehabilitation
in Diabetes, Endocrine and Metabolic Disorders.