Research Article :
Mahfuj-Ul-Anwar,
Sajeda Afrin, ASM Rahenur Mondol, Mohammad Nurul Islam
Khan, Narayan Chandra Sarkar, Mohammad Kumruzzaman Sarker,
Shah Md Sarwer Jahan, Moni Rani and Ratindra Nath Mondal Background: Stroke is a leading cause of mortality and
disability worldwide. To prevent complications and permanent defects, early
diagnosis, distinguishing the type and risk factor of stroke is crucial. Methodology: It was a hospital based
cross sectional study, purposive sampling method was used, and a total of 469
stroke patients admitted into Department of Medicine, Rangpur medical college
hospital, Bangladesh were included in this study. Results: In this study we have studied of 469 acute stroke
patients. Among them 81% (380) were ischemic stroke patients and 19% (89) were
hemorrhagic stroke. Overall male were more than female 308 (65.7%) vs
161(34.4%). The mean age for the ischemic stroke group was 64.1 ± 10.9 years,
which was significantly higher than that of the hemorrhagic group (59.8 ± 9.60years)
(P<0.05). Acute hemorrhagic stroke patients presented with acute onset of
focal neurological deficit 61.8%, headache 64%, vomiting 59.6%, alteration of
consciousness 48.3% and convulsion 27%. On the other hand, acute ischemic
stroke patient presented with alteration of consciousness 65.5%, acute onset of
focal neurological deficit 39.5%, paralysis 41%, deficit after awakening 32.4%
and aphasia 34.7%. Among the risk factors of stroke in acute ischemic stroke
patients hypertension was 59.2%, diabetes mellitus 20%, history of previous
stroke 16.1%, ischemic heart disease 14.5% and atrial fibrillation 10.3% were
present, on the other hand in acute hemorrhagic stroke patients hypertension
76.4%, smoking 70.8% and diabetes mellitus 6.7% were present. 26.97% of the
acute hemorrhagic stroke and 13.9% of the acute ischemic stroke patients died
in hospital. Conclusion: Common
presentation of stroke was acute onset of focal neurological deficit; headache
and vomiting were more in hemorrhagic stroke patient; alteration of
consciousness, paralysis was predominant in ischemic stroke patient Stroke
was defined according to WHO criteria as rapidly developing clinical signs of
focal (at times global) disturbance of cerebral function lasting more than 24 hours
or leading to death with no apparent cause other than that of vascular origin.
Two types of brain stroke are hemorrhagic and ischemic. Hemorrhagic stroke,
which is due to blood vessel rupture, accounts for 20% of CVAs. Ischemic stroke
due to brain vessels occlusion and blockage includes 80%. Stroke is a leading
cause of mortality and disability worldwide and the economic costs of treatment
and post-stroke care are substantial. Results from the 2015 iteration of the
Global Burden of Diseases (GMD), Injuries, and Risk Factors Study (RFS) showed
that although the age-standardized death rates and prevalence of stroke have
decreased over time, the overall burden of stroke has remained high, with more
than 80 million [1-4]. Stroke
survivors in 2016. In 2016, stroke was the second largest cause of death globally
(5.5 million deaths) after ischemic heart disease. Stroke was also the second
most common cause of global Disability-Adjusted Life-Years DALYs (116.4
million). There were 80.1 million prevalent cases of stroke globally in 2016
and 13.7 million new stroke cases in 2016. In 2016 the number of stroke patient
and death due to stroke in Bangladesh were 161,709 and 126,369 respectively. In
order to prevent complications and permanent defects, early diagnosis is the
key in stroke patients; however, distinguishing the type of stroke plays a
crucial role in patient care. The management of a patient with acute stroke is
based on the knowledge of stroke type: hemorrhagic or ischemic. In most
developed countries, diagnosis is easily obtained by CT scanning, which allows
the accurate Distinction of hemorrhagic and ischemic types [5,6]. However,
quick access to CT scanning is not available in every country and hospital
which may lead to loss of treatment golden time. Simple clinical findings are
helpful in distinguishing the type of stroke, but need for diagnostic imaging is
an undeniable fact. According to this issue, many studies described various
clinical findings especially neurological signs and symptoms and risk factors
differentiation, and some of them presented formulas to distinguish stroke
types based on clinical evaluations. As populations age and low-income and
middle-income countries go through the epidemiological transition from
infectious to non-communicable diseases as the predominant cause of morbidity,
together with concomitant increases in modifiable risk factors, it is expected
that the burden of stroke will further increase until effective stroke
prevention strategies are more widely implemented [7-14]. About
90% of the stroke burden is attributable to modifiable risk
factors, with about 75% being due to behavioral factors such as smoking,
poor diet and low physical activity. Achieving control of behavioral and
metabolic risk factors could avert more than three quarters of the global
stroke burden. Since treatment measures for stroke are still rather limited and
expensive, in a high prevalent country like Bangladesh, it is important to be
familiar with relative contribution of different stroke risk factor in an
individual patient. The individuals with a relatively high risk profile can
take steps to modify their risk factors through lifestyle changes and/or
medical treatment. Healthy lifestyle modification and better adherence to
recommended medications via an affordable multidrug
polypill containing blood pressure and lipid-lowering medications, early
initiation of antiplatelet drugs after ischemic stroke could potentially also
enable cost-effective prevention of stroke globally, potentially halving stroke
incidence and mortality [15-18]. In
addition to prevention efforts, appropriate acute and long-term treatment is
essential, given the high recurrence rate of stroke. Similarly, public
awareness programs aimed at increasing the recognition of stroke warning signs
and altering modifiable risk factors can be designed to address the high-risk
groups. Because the pathogenesis of ischemic stroke is different from that of
hemorrhagic stroke, their clinical factors including risk factors would not be
the same. This study was undertaken to assess the difference in risk factors,
clinical and laboratory profiles in hemorrhagic and ischemic stroke patients so
as to provide some scientific evidence for stroke prevention in Bangladesh
[19-21]. This
was a hospital based cross sectional study was conducted in Department of
Medicine, Rangpur medical college hospital, Rangpur, Bangladesh from January
2010 to December 2011. Purposive sampling method was used. The study included
469 patients with acute stroke diagnosed by history, clinical findings and
confirmed by CT scan of brain within 1week of attack. Patients with no
definitive CT scan results or those suspected to have transient ischemic
attacks were excluded from the study. For each patient, demographic data, type
of stroke, risk factors, clinical and initial laboratory variables and
in-hospital death were recorded. Demographic variables included age and sex. Types
of stroke included ischemic and hemorrhage. Risk
factors included a history of Hypertension (HTN), Diabetes Mellitus (DM), Ischemic
Heart Disease (IHD), Valvular
Heart Disease (VHD), Atrial Fibrillation (AF), Renal Impairment (RI), Smoking,
Obesity (SO), advanced age (>80 years), previous stroke and family history
of stroke, hypertension, Diabetes Mellitus (DM) and Coronary Artery Disease (CAD).
Patient outcome included vital status at discharge (alive or dead). All
patients were investigated with routine investigations such as TC, DC, ESR,
Hb%, total platelet count, urine examination, RBS (on admission blood sugar
level), FBS and 2HABF, fasting lipid profile, serum electrolytes, serum
creatinine, ECG. CT scan of brain was done in every case to confirm the
diagnosis. Treatment was given accordingly and inpatient outcome were observed.
The clinical factors to be observed in this study included advanced age (>80
years), gender (male-exposure), cigarette smoking (average smoking ≥ 1
cigarettes per day, and continued more than one year). Hypertension was
diagnosed when the blood pressure measured in the hospital was >140/90 mmHg
or if the patient was taking antihypertensive agents. Diabetes was diagnosed if
a patient was using oral hypoglycemic agents or insulin and or post-stroke
repeated fasting plasma glucose levels ≥ 7.0 mmol/L (≥ 126 mg/dL) and 2 hours
after glucose level ≥ 11.1mmol/L (≥ 200mg/dl) and or the glycosylated
hemoglobin level exceeded 6.5%. Obesity
was defined based on the body mass index (BMI) value; males and females with
BMI >30 were considered to be obese. On admission laboratory reports
includes increased white blood cell (WBC>11.0×109/L), hypertriglyceridemia
(triglyceride (TG)>1.7 mmol/L), hypercholesterolemia (total cholesterol (TC)
≥ 5.7 mmol/L), low level of high-density lipoproteins (HDL<1.0 mmol/L), ischemic
changes or arrhythmia on ECG. All relevant information was recorded in a
predesigned questionnaire. Collected data were compiled and appropriate
analyses were carried out using computer-based software, Statistical Package
for Social Science (SPSS)-17. The continuous clinical variants were compared by
unpaired Student's t test. The Chi-square test was used to evaluate differences
in proportion of clinical factors in patients between ischemic and hemorrhagic
stroke. A P value <0.05 (two-tailed) was considered statistically
significant. Table 1: Comparison of the age of patients (in percentage) with ischemic and hemorrhagic stroke. Acute
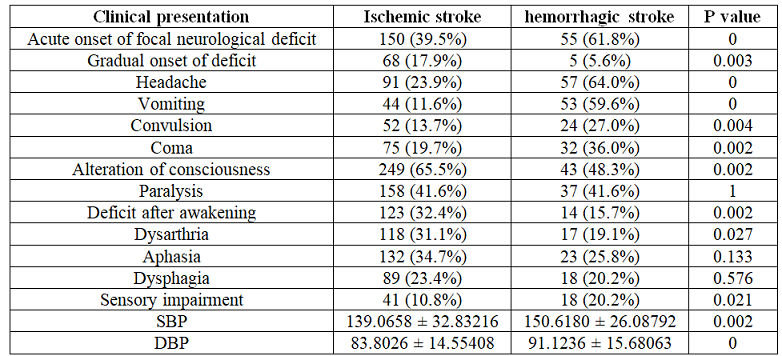
hemorrhagic stroke patients presented with acute onset of focal neurological
deficit 61.8%, headache 64%, vomiting 59.6%, alteration of consciousness 48.3%
and convulsion 27%. On the other hand, acute ischemic stroke patient presented
with alteration of consciousness 65.5%, acute onset of focal neurological
deficit 39.5%, paralysis 41%, deficit after awakening 32.4% and aphasia 34.7%.
Clinical
presentations of two subtypes of stroke were detailed in (Table 2). Among
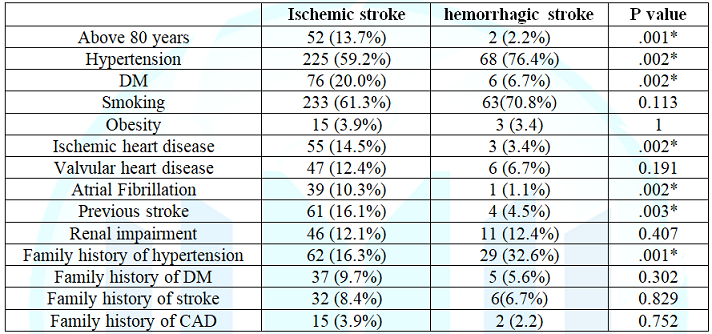
the risk factors of stroke in acute ischemic stroke patients hypertension was
59.2%, diabetes
mellitus 20%, history of previous stroke 16.1%, ischemic heart disease
14.5% and atrial fibrillation 10.3% were present, on the other hand in acute
hemorrhagic stroke patients hypertension 76.4%, smoking 70.8% and diabetes
mellitus 6.7% were present. (Table 3)
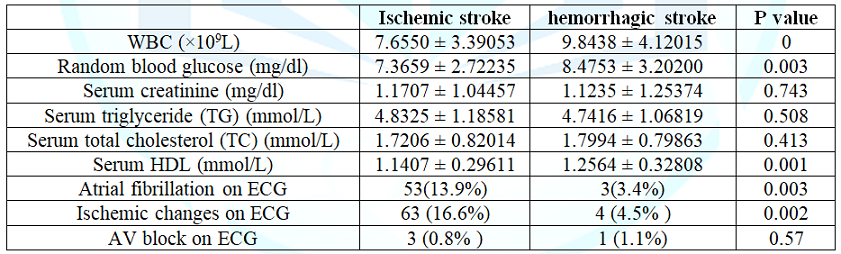
showing risk factors of the ischemic and hemorrhagic stroke) the laboratory
data of patients with ischemic and hemorrhagic stroke were compared at (Table 4). Atrial fibrillation and
ischemic changes on ECG was more in ischemic stroke than hemorrhagic stroke,
13.9% vs 3.4% and 16.6% vs 4.5% respectively. 26.97% of the acute hemorrhagic
stroke and 13.9% of the acute ischemic stroke patients died in hospital. Table 2: Comparison of clinical presentations on admission of ischemic and hemorrhagic stroke. Table 3: Comparison of risk factors of the ischemic and hemorrhagic stroke. In
our study 81% patients were ischemic stroke, similar findings observed in other
studies. Observed higher rates of ischemic stroke incidence suggests that
ischemic stroke patients have a great exposure to modifiable risk factors which
can be controlled through lifestyle modification and appropriate treatment thus
can prevent a large proportion of such incidence of stroke. Stroke (both
ischemic and hemorrhagic) is more common in men than women, our study also
found similar result. Lifestyle differences, such as cigarette smoking and
alcohol drinking, may help explain this sex disparity. In addition, there is no
vascular protection of endogenous estrogen in males and it may contribute to
the risk of stroke in men. In the current study, mean age of patients with
ischemic stroke was higher than hemorrhagic
stroke patients, similar findings observed in many of the other studies Knowledge
on the relative contribution of risk factors in hemorrhagic versus ischemic
strokes is still insufficient. Some risk factors are common for both hemorrhagic
and ischemic stroke. In our study factors favoring ischemic stroke as opposed
to hemorrhagic [22-29]. Strokes
were diabetes, atrial fibrillation, history of previous stroke and increasing
age. Hypertension and family history of hypertension favored hemorrhagic stroke
as opposed to ischemic stroke. In our study smoking, obesity, Valvular Heart
Disease (VHD), renal impairment, family history of DM, stroke or coronary
artery disease favored neither of the stroke types. In a large study based on
394, 84 patients’ well-established risk factors and markers of atherosclerotic
and occlusive arterial disease such as diabetes, atrial fibrillation, previous
myocardial infarction, previous stroke and intermittent arterial claudication
were associated with ischemic stroke rather than hemorrhagic stroke smoking and
high alcohol intake favored hemorrhagic stroke, whereas age, sex, and
hypertension did not herald stroke type. In the hospital-based Lausanne Stroke
registry (n=3901) smoking, hypercholesterolemia, migraine, previous transient ischemic
attack, atrial fibrillation, and heart disease favored ischemic stroke, whereas
hypertension was the only significant factor related to hemorrhagic vs ischemic
stroke. Hypertension is the most prevalent risk factor for stroke, based on
data from 30 studies, and has been reported in about 64% of patients with
stroke. High blood pressure can [30-33]. significantly increase the risk of a
hemorrhagic stroke. This risk is even more pronounced in the elderly, in people
who smoke, in men, in diabetics, and in people who drink alcohol. In our study
59.2% of the ischemic
stroke and 76.4% of the hemorrhagic stroke patient had hypertension. Recent
large-scale, international population studies suggest that diabetes is one of
the most Important modifiable risk factors for cerebrovascular disease.
Diabetes is also a well-established independent risk factor for ischemic stroke.
Diabetes causes various microvascular and macrovascular changes often Cerebral
Small Vessel Diseases (CSVD) and ultimately develops ischemic stroke. Cigarette
smoking has long been recognized as major risk factors for stroke. The
pathophysiological effects are multifactorial, involving both systemic vasculature
and blood rheology. So far it is still controversial whether the effects of
cigarette smoking on ischemic stroke are consistent with those on hemorrhagic
stroke (Clinical factors in patients with ischemic versus hemorrhagic stroke in
East China). The data from our study exhibited that the association of smoking
with hemorrhagic stroke was approximately the same as that with ischemic
stroke, similar result also found in previous studies [34-45]. Dyslipidemia
have traditionally been regarded as a risk factor for coronary artery disease
but not for cerebrovascular disease. However, recent studies have clarified the
relationship between lipids and ischemic stroke, and showed that the risk of
ischemic stroke. In a large cohort of elderly patients, low triglycerides
levels were associated with an increased risk of hemorrhagic stroke. In our
study, we observed a low level of Clinical
features, such as acute onset, headache, vomiting, convulsion, increased
systolic and diastolic blood pressure on admission, decreased consciousness and
coma were significant in patients with hemorrhagic stroke than ischemic stroke
patients reported by others our study also found similar result. Severity of
the clinical features in hemorrhagic stroke presumably due to increased
intracranial pressure and the direct compression or distortion of the thalamic
and brain-stem reticular activating system, expansion of hematoma, worsening
cerebral edema and meningismus resulting from blood in ventricles. On the other
hand gradual onset, deficit after awakening, focal deficits, visual field
defect and sensory impairment were significant in patients with ischemic stroke
also observed previously, however, occurrence site of these signs depends on
the brain area that is being nourished by suffering vessels. On initial investigations
like previous studies WBC, Blood Glucose (BG), HDL, were higher in the
hemorrhagic group than in the ischemic group. On the other hand ischemic
changes and atrial fibrillation8 on ECG is significantly observed in ischemic
stroke patients. Our study findings are consistent with all those studies.
Moreover, a previous study reported a significant elevation of the WBC count in
ICH was associated with deteriorating level of consciousness, cerebral
vasospasm and death. Increased WBC count is mainly attributed to enhanced
catecholamine and corticosteroid release because of an extension of the blood
into the subarachnoid space, but the inflammatory response of ventricular
extension (ventriculitis) may be an additional contributing. Factor So, WBC
count may. Be one of the important prognostic markers in hemorrhagic stroke
patients. Hospital mortality is higher in hemorrhagic stroke than ischemic
stroke. Ratindra et al found short term. (Within 28 days) mortality in
hemorrhagic stroke 45.5%, where hospital mortality was 40% and in ischemic
stroke 18.1% and hospital mortality was 7.97%. Our study found similar result [51-61].
Proper
stroke management depends on the distinction between intracerebral hemorrhage
and cerebral infarction. Whilst CT imaging remains the gold standard for
differential diagnosis, availability of this important diagnostic tool is not
always feasible. Despite the lack of absolute accuracy of classification
models, adequate knowledge on risk factors, clinical features and initial
investigations may contribute to such a differentiation of cerebral infarction
from intracerebral
hemorrhage in order to aid clinicians to decide about starting antiplatelet
therapy in settings where rapid access to Computed Tomography (CT) is lacking
[8]. Nevertheless, a combined analysis of 40000 randomized patients from the
Chinese Acute Stroke Trial (CAST) has demonstrated that early aspirin use
(within 48 hours of onset) amongst the 9000 patients (22%) randomized without a
prior CT scan appeared to be of net benefit with no unusual excess of
hemorrhagic stroke. Moreover, even amongst the 800 subjects (2%) whose
presenting event was subsequently discovered to have been a hemorrhagic stroke,
there was no evidence of detrimental effect of aspirin (OR 0.86 for further
stroke or death, 63 aspirin versus 67 control). Because in CAST and IST trials
the incidence of hemorrhagic stroke or transformation was low during the first
day from the event, whereas that of recurrent ischemic stroke was relatively
high, early aspirin use is justifiable when ischemic stroke is suspected and
rapid CT scanning is lacking [62-64].Clinical Features, Risk Factors and Hospital Mortality of Acute Stroke Patients
Abstract
Full-Text
Introduction
Materials
and Methods
Results
In
this study we have studied of 469 acute stroke patients. Among them 81% (380)
were ischemic stroke patients and 19% (89) were hemorrhagic stroke. Overall
male were more than female 308 (65.7%) vs 161 (34.4%) and also in both types of
stroke patients (ischemic stroke group 65.3% and 67.4% of the hemorrhagic
group). The mean age for the ischemic stroke group was 64.08 ± 10.89 years,
which was significantly higher than that of the hemorrhagic group (59.82 ± 9.60years)
(P<0.05). (Table 1) shows the details.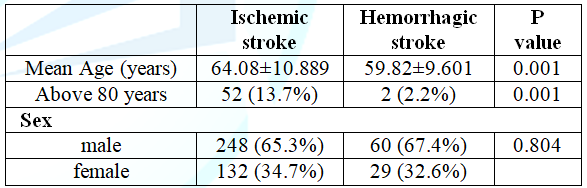
Discussion
Conclusion
Common presentation of stroke was acute onset of
focal neurological deficit; headache and vomiting was more in hemorrhagic
stroke patient; alteration of consciousness, paralysis was predominant in
ischemic stroke patient.
1. Aho
K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, et al. Cerebrovascular
disease in the community: results of a WHO collaborative study (1980) Bull
World Health Organ 58: 113-130.
2. Marx
J, Hockberger R, Walls R. Rosen's Emergency Medicine (2006) Elsevier, Netherlands
3. Tntinalli
JE, Kelen GD and Stapczynski JS. Emergency Medicine: A Comprehensive Study
Guide (2004) Mc raw-hill, USA
4. Rajsic
S, Gothe H, Borba HH, Sroczynski G, Vujicic J, et al. Economic burden of
stroke: a systematic review on post-stroke care (2018) Eur J Health Econ 20:
107-134.
https://doi.org/10.1007/s10198-018-0984-0 .
5. Feigin
VL, Abajobir AA, Abate KH, Abd-Allah F, Abdulle AM, et al. Global, regional,
and national burden of neurological disorders during 1990-2015: a systematic
analysis for the global burden of disease study 2015 (2017) Lancet Neurol 16:
877-897. https://doi.org/10.1016/S1474-4422(17)30299-5
6. Johnson
COJ, Nguyen M, Roth EA, Nichols E, Alam T, et al. Global, regional, and national
burden of stroke, 1990-2016: a systematic analysis for the Global Burden of
Disease Study 2016 (2019) Lancet Neurol 18: 439-458.
https://doi.org/10.1016/S1474-4422(19)30034-1
7. Besson
G, Robert C, Hommel M and Perret J. Is it clinically possible to distinguish
nonhemorrhagic infarct from hemorrhagic stroke? (1995) Stroke 26: 1205-1209.
https://doi.org/10.1161/01.
STR.26.7.1205
8. Efstathiou
SP, Tsioulos DI, Zacharos ID, Tsiakou AG, Mitromaras AG, et al. A new
classification tool for clinical differentiation between haemorrhagic and
ischaemic stroke (2002) J Intern Med 52: 121-129. https://doi.org/10.1046/j.1365-2796.2002.01013.x
9. Ojaghihaghighi
HS, Vahdat SS, Mikaeilpour A and Ramouz A. Comparison of neurological clinical
manifestation in patients with hemorrhagic and ischemic stroke (2017) World J
Emerg Med 8: 34-38. https://dx.doi.org/10.5847%2Fwjem.j.1920-8642.2017.01.006
10. Williams GR, Jiang JG, Matchar DB and Samsa GP. Incidence and occurrence of total (first-ever and recurrent) stroke (1999) Stroke 30: 2523-2528.
https://doi.org/10.1161/01.str.30.12.2523
11. Smith RW, Scott PA, Grant RJ, Chudnofsky CR and Frederiksen SM. Emergency physician treatment of acute stroke with recombinant tissue plasminogen activator: a retrospective analysis (1999) Acad Emerg Med 6: 618-625.
12. Lewandowski
CA, Frankel M, Tomsick TA, Broderick J, Frey J, et al. Combined intravenous and
intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: emergency
management of stroke (EMS) bridging trial (1999) Stroke 30: 2598-2605.
https://doi.org/10.1161/01.str.30.12.2598
13. Khan
J and Rehman A. Comparison of clinical diagnosis with computed tomography in
ascertaining type of stroke (2005) J Ayub Med Coll Abbottabad 17: 65-67.
14. Celani
MG, Righetti E, Migliacci R, Zampolini M, Antoniutti L, et al. Comparability
and validity of two clinical scores in the early differential diagnosis of
acute stroke (1994) BMJ 308: 1674-1676. https://dx.doi.org/10.1136%2Fbmj.308.6945.1674
15. Yan
LL, Li C, Chen J, Luo R, Bettger J, et al. Cardiovascular, respiratory, and
related disorders (2017) Prabhakaran D (ed), Anand S (ed), Gaziano TA (ed). USA
16. Wajngarten
M and Sampaio GS. Hypertension and Stroke: Update on Treatment (2019) Eur
Cardiol 14: 111-115. https://dx.doi.org/10.15420%2Fecr.2019.11.1
17. Feigin
VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, et al. Global burden of
stroke and risk factors in 188 countries, during 1990-2013: a systematic
analysis for the global burden of disease study 2013 (2016) Lancet Neurol 15:
913-1924. https://doi.org/10.1016/S1474-4422(16)30073-4
18. Riaz
BK, Chowdhury SH, Karim MN, Feroz S, Selim S, et al. Risk factors of
hemorrhagic and ischemic stroke among hospitalized patients in Bangladesh a
case control study (2015) Bangladesh Med Res Counc Bull 41: 29-34.
19. Feigin VL, Norrving B, George MG, Foltz JL, Roth GA, et al. Prevention of stroke: a strategic global imperative (2016) Nat Rev Neurol 12: 501-512.
20. Gaziano
TA, Opie LH and Weinstein MC. Cardiovascular disease prevention with a
multidrug regimen in the developing world: a cost-effectiveness analysis (2006)
Lancet 368: 679-686.
https://doi.org/10.1016/s0140-6736(06)69252-0
21. Brainin M, Feigin V, Martins S, Matz K, Roy J, et al. Cut stroke in half: polypill for primary prevention in stroke (2018) Int J Stroke 13: 633-647.
https://doi.org/10.1177/1747493018761190
22. Zhang J, Wang Y, Wang GN, Sun H, Shi JQ, et al. Clinical factors in patients with ischemic versus hemorrhagic stroke in East China (2011) World J Emerg Med 2: 18-23.
23. Andersen
KK, Olsen TS, Dehlendorff C and Kammersgaard LP. Hemorrhagic and ischemic
strokes compared stroke severity, mortality, and risk factors (2009) Stroke 40:
2068-2072.
https://doi.org/10.1161/strokeaha.108.540112
24. Mozaffarian
D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke
statistics-2015 update: a report from the American Heart Association (2015)
Circulation 131: e29-e322.
https://doi.org/10.1161/CIR.0000000000000152
25. Riaz
BK, Chowdhury SH, Karim MN, Feroz S, Selim S, et al. Risk factors of hemorrhagic
and ischemic stroke among hospitalized patients in Bangladesh a case control
study (2015) Bangladesh Med Res Counc Bull 41: 29-34.
https://doi.org/10.3329/bmrcb.v41i1.30231
26. Massaro
AR, Sacco RL, Scaff M and Mohr JP. Clinical discriminators between acute brain hemorrhage
and infraction (2002) Arq Neuropsiquiatr 60: 185-191.
https://doi.org/10.1590/S0004-282X2002000200001
27. Perna
R and Temple J. Rehabilitation outcomes: ischemic versus hemorrhagic strokes (2015)
Behavioural Neurology 2015: 891651.
http://dx.doi.org/10.1155/2015/891651
28. Oh JS, Bae HG, Oh HG, Yoon SM, Doh JW, et al. The changing trends in age of first-ever or recurrent stroke in a rapidly developing urban area during 19 years (2017) J Neurol Neurosci 2: 206.
http://dx.doi.org/10.21767/2171-6625.1000206
29. Benjamin
EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, et al. Heart disease and stroke
statistics-2017 update: a report from the American Heart Association (2017) Circulation
135: e146-e603.
https://doi.org/10.1161/CIR.0000000000000485
30. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, et al. Spontaneous intracerebral hemorrhage (2001) N Engl J Med 344: 1450-1460.
https://doi.org/10.1056/NEJM200109063451014
31. Ferro JM. Update on cerebral haemorrhage (2006) J Neurol 253: 985-999.
https://doi.org/10.1007/s00415-006-0201-4
32. Ha¨nggi D and Steiger HJ. Spontaneous intracerebral haemorrhage in adults: a literature overview (2008) Acta Neurochir 150: 371-379.
https://doi.org/10.1007/s00701-007-1484-7
33. Liu
XF, van Melle G and Bogousslavsky J. Analysis of risk factors in 3901 patients
with stroke (2005) Chin Med 20: 35-39.
34. Feigin
VL, Norrving B and Mensah GA. Global burden of stroke (2017) Circ Res 120:
439-448.
https://doi.org/10.1161/circresaha.116.308413
35. An SJ, Kim TJ and Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update (2017) J Stroke 19: 3-10.
https://dx.doi.org/10.5853%2Fjos.2016.00864
36. Harvard Medical
School. Hemorrhagic stroke. February 2019.
37. Burton JK, Quinn TJ and Fisher M. Diabetes and stroke (2019) 36: 4.
https://doi.org/10.1002/pdi.2230
38. The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies (2010) Lancet 375: 2215-2222.
https://doi.org/10.1016/S0140-6736(10)60484-9
39. Arboix
A, Morcillo C, García-Eroles L, Massons J, Oliveres M, et al. Different
vascular risk factor profiles in ischemic stroke subtypes: The SagratCor Hospital
of Barcelona Stroke Registry (2000) Acta Neurol Scand 102: 264-270.
https://doi.org/10.1034/j.1600-404.2000.102004264.x
40. Arabadzhieva
D, Kaprelyan A, Georgieva Z, Tsukeva A, et al. Diabetes mellitus as a risk
factor for ischemic stroke (2014) Science and Technologies 4: 27-30.
41. Khoury
JC, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, et al. Diabetes mellitus: a risk
factor for ischemic stroke in a large biracial population (2013) Stroke 44: 1500-1504.
https://doi.org/10.1161/strokeaha.113.001318
42. Shi Y and Wardlaw JM. Update on cerebral small vessel disease: a dynamic whole-brain disease (2016) Stroke Vasc Neurol 1: 83-92.
https://dx.doi.org/10.1136%2Fsvn-2016-000035
43. Ariesen
MJ, Claus SP, Rinkel GJE and Algra A. Risk factors for intracerebral hemorrhage
in the general population: a systematic review (2003) Stroke 34: 2060-2066.
https://doi.org/10.1161/01.str.0000080678.09344.8d
44. Kurth
T, Kase CS, Berger K, Schaeffner ES, Buring JE, et al. Smoking and the risk of
hemorrhagic stroke in men (2003) Stroke 34: 1151-1155.
https://doi.org/10.1161/01.STR.0000065200.93070.32
45. turgeon
JD, Folsom AR, Longstreth WT, Shahar E, Rosamond WD, et al. Risk factors for
intracerebral hemorrhage in a pooled prospective study (2007) Stroke 38: 2718 -2725.
https://doi.org/10.1161/strokeaha.107.487090
46. de
Craen AJ, Blauw GJ, Westendorp RG. Cholesterol and risk of stroke: cholesterol,
stroke, and age (2006) BMJ 333: 148.
https://dx.doi.org/10.1136%2Fbmj.333.7559.148
47. Smith
EE, Abdullah AR, Amirfarzan H and Schwamm LH. Serum lipid profile on admission
for ischemic stroke: failure to meet national cholesterol education program
adult treatment panel (NCEP2ATPIII) guidelines (2007) Neurology 68: 660-665. https://doi.org/10.1212/01.wnl.0000255941.03761.dc
48. Bonaventure
A, Kurth T, Pico F, Barberger-Gateau
P, Ritchie K, et al. Triglycerides and risk of hemorrhagic stroke vs. ischemic
vascular events: The Three-City Study (2010) Atherosclerosis 210: 243-248.
https://doi.org/10.1016/j.atherosclerosis.2009.10.043
49. Huisman
MV, Lip GY, Diener HC, Halperin JL, Rothman KJ, et al. Design and rationale of
Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with
Atrial Fibrillation: a global registry program on long-term oral antithrombotic
treatment in patients with atrial fibrillation (2014) Am Heart J 167: 329-334.
https://doi.org/10.1016/j.ahj.2013.12.006
50. Hart
RG, Pearce LA and Aguilar MI. Meta-analysis: antithrombotic therapy to prevent
stroke in patients who have nonvalvular atrial fibrillation (2007) Ann Intern
Med 146: 857-867.
https://doi.org/10.7326/0003-4819-146-12-200706190-00007
51. Aring CD and Merritt HH. Differential diagnosis between cerebral hemorrhage and cerebral thrombosis: a clinical and pathologic study of 245 cases. (1935) Arch Intern Med 56: 435-456.
https://doi.org/10.1001/archinte.1935.00170010023002
52. Bogousslavsky
J, van Melle G and Regli F. The Lauanne Stroke Registry: analysis of 1000
consecutive patients with first stroke (1988) Stroke 19: 1083-1092.
https://doi.org/10.1161/01.str.19.9.1083
53. Mohr
JP, Caplan LR, Melski JW, Goldstein
RJ, Duncan GW, et al. The harvard cooperative stroke registry: a prospective
registry (1978) Neurology 28: 754-762.
https://doi.org/10.1212/wnl.28.8.754
54. Melo
TP, Pinto AN and Ferro JM. Headache in intracerebral hematomas (1996) Neurology
47: 494-500.
https://doi.org/10.1212/wnl.47.2.494
55. Davis PH, Dambrosia JM, Schoenberg BS, Schoenberg DG, Pritchard DA, et al. Risk factors for ischemic stroke: a prospective study in Rochester, Minnesota (1987) Ann Neurol 22: 319-327.
https://doi.org/10.1002/ana.410220307
56. Fogelholm R, Murros K, Rissanen A and Ilmavirta M. Factors delaying hospital admission after acute stroke (1996) Stroke 27: 398-400.
https://doi.org/10.1161/01.STR.27.3.398
57. Kothari R, Hall K and Brott T. Early stroke recognition: developing an out-of-hospital NIH Stroke Scale (1997) Acad Emerg Med 4: 986-990.
https://doi.org/10.1111/j.1553-2712.1997.tb03665.x
58. Dávalos A, Castillo J, Martinez-Vila E, Delay in neurological attention and stroke outcome cerebrovascular diseases study group of the Spanish society of neurology (1995) Stroke 26: 2233-2237.
59. Jorgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS, et al. Factors delaying hospital admission in acute stroke: the Copenhagen Stroke Study (1996) Neurology 47: 383-387.
60. Neil-Dwyer G and Cruickshank J. The blood leukocyte count and its prognostic significance in subarachnoid haemorrhage (1947) Brain 97: 79-86.
https://doi.org/10.1093/brain/97.1.79
61. Suzuki S, Kelley RE, Dandapani BK, Reyes-Iglesias Y, Dietrich WD, et al. Acute leukocyte and temperature response in hypertensive intracerebral haemorrhage (1995) Stroke 26: 1020-1023.
https://doi.org/10.1161/01.str.26.6.1020
62. Mondal
RN, Barman S, Islam MJ, Jahan SMS, Alam ABMM, et al. Short-term predictors of
mortality among patients with hemorrhagic stroke (2014) World Heart J 6: 273-282.
63. Mondal
RN, Jamal M, Islam MA, Anwar MM, Rahman MM, et al. Short term outcome of acute
ischemic stroke patients (2020) EC Cardiology 7: 10-18.
64. Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, et al. Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40000 randomized patients from the Chinese acute stroke trial and the international stroke trial (2000) Stroke 31: 1240-1249.
https://doi.org/10.1161/01.str.31.6.1240
Dr. Ratindra Nath Mondal, Society of General Physicians and Daktarkhana (GP
center) Rangpur, Bangladesh, Email: dr.ratinmondal@gmail.com
Anwar-Ul-M, Afrin S, Mondol RASM, Khan MNI, Sarkar CS, et al. Clinical features, risk factors and hospital mortality of acute stroke patients (2020) J Obesity and Diabetes 4: 9-14.
Ischemic, Hemorrhagic, Stroke, Rangpur, Bangladesh.