Review Article :
Guillain-BarréSyndrome (GBS) is an acute, monophasic, autoimmune polyradiculoneuropathy,
described just over a century ago, and remains an important cause of
neuromuscular paralysis worldwide [1-4]. The clinical presentation of GBS is
heterogeneous and can range from a mild self-limiting muscle weakness to a
life-threatening quadriplegia with respiratory failure necessitating artificial
ventilation. There is an increasing awareness of the diverse range of not only
clinical, but also electrophysiological and autoantibody profiles that
characterize GBS, suggesting that it is not a singular condition, but rather a
spectrum of related disorders [5-7]. The aim of this article is to provide an overview, and an update, of GBS, with
discussions pertaining to its epidemiology, aetiology, clinical presentation,
investigation, diagnosis, management and prognosis of this acute neurological
disorder. Most
epidemiological studies on GBS have been undertaken in Europe and North
America. The overall annual incidence of GBS is estimated to be 1-2/100,000 per
year [2] though this figure rises with age above 50 years to up to 3.3/100 000
per year [2,4] and men are more frequently affected than women (3:2) across all
ages [2]. Various epidemiological studies have also demonstrated a bimodal age
distribution in incidence of GBS, although there is some disagreement between
studies and the age categories in which these peak incidences, if they are
identified, are variable [8-13]. GBS
exists in both demyelinating (Acute Inflammatory Demyelinating Polyradiculoneuropathy
[AIDP]) and axonal (Acute Motor Axonal Neuropathy [AMAN] and Acute Motor and
Sensory Axonal Neuropathy [AMSAN]) forms [14-17]. The recent International GBS
Outcome Study (IGOS) has shown that geographical location exerts a major
influence in GBS clinical phenotype, disease severity and patient outcomes, but
also electrophysiological subtype [15]. AIDP was the predominant subtype in all
regions investigated (Europe/Americas: 55%, Asia: 45%, Bangladesh: 40%),
whereas the axonal subtype was more frequent in Bangladesh (36%) than in
Europe/Americas (6%) and other Asian countries (6%) [15]. In all regions
assessed, patients with the axonal subtype showed a trend towards poorer
recovery [15]. GBS is an immune-mediated disorder preceded by respiratory infection or
gastroenteritis (classically by CampylobacterJejuni), within 4 weeks of onset of muscle weakness, in nearly two-thirds
of adult patients [18]. The occurrence of such prodromal illnesses may also
explain seasonal fluctuations in the incidence of GBS cases, though this has
only been reported in certain geographical regions [19]. In more extreme case
scenarios, GBS has been demonstrated to closely track infectious outbreaks
spatially and temporally. This has been
seen in outbreaks of C. Jejuni
infection in North America [20] and also more recently highlighted by the
dramatic rise in incidence of GBS cases in Brazil and Colombia in 2015-2016
following the Zika virus outbreak
[21,22]. Thus, the epidemiology of GBS is dynamic and at least partly sculpted
by the incidence and distribution of certain antecedent -infective illnesses. Many
microbial causes have been implicated in antecedent infection preceding GBS.
These include Influenza A virus, Cytomegalovirus, Epstein-Barr virus, MycoplasmaPneumoniae, Hepatitis E and more
recently Zika virus [18,22]. However,
the commonest antecedent infective cause is C.
Jejuni, which has been linked to the axonal variant of GBS [23]. Precisely
why less than 0.1% of patients with C.
Jejuni enteritis develop GBS within the following 2 months is not entirely
clear [24]. This may be due to a combination of host genetic susceptibility
factors and infection with subtype-specific strains of this bacterium, which
can be highly variable [25]. Similar
principles may be relevant with other microbes. An important mechanism underlying the aetio-pathogenesis of GBS is believed to
be molecular mimicry, whereby antibodies generated by the host, against target
microbial antigens, cross-react with neural epitopes. In the case of GBS
following C. Jejuni infection,
antibodies cross-react with certain ganglioside antigens clustered on axonal
membranes, such as GD1a or GM1, resulting in the AMAN GBS variant [23,25].
Anti-ganglioside antibody generation also occurs in association with complement
activation, which further drives peripheral nerve degeneration. Indeed,
blocking complement can be neuro-protective in mouse models of GBS [26]. In
classic cases, GBS presents as an acute, ascending, symmetrical, flaccid muscle
paralysis, which can progress over the course of days to several weeks, to
quadriplegia with or without cranial nerve involvement. Involvement of diaphragmatic and intercostal
muscles may lead to respiratory failure requiring intensive care support and
invasive mechanical ventilation, in up to 20-30% of hospitalized patients,
which is usually associated with a poor outcome and significant mortality [27-31]. GBS is a monophasic illness, which reaches nadir within 4 weeks in the majority
of patients, but typically within 2 weeks [32]. If there is clinical
progression beyond 4 weeks, then this should suggest an alternative diagnosis,
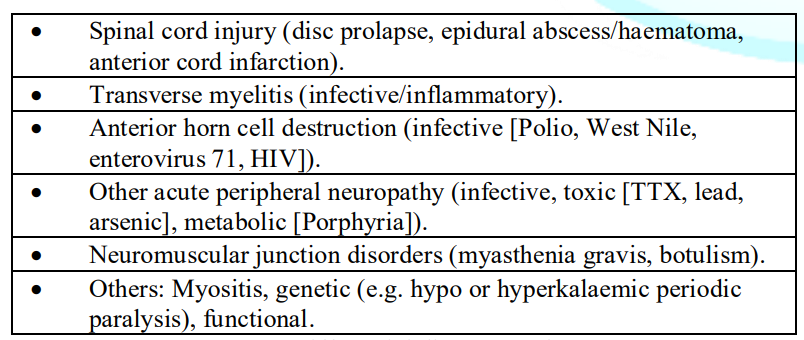
(although 3% can progress to week 6) [32]. Table
1 lists the differential diagnoses of GBS. Table 1: Differential
diagnoses of GBS Despite
usually being recognized as a disease restricted to lower motor neurons, with
hypo- or areflexia, approximately 10% of patients have normal or brisk deep
tendon reflexes, suggesting that concomitant upper motor neuron involvement
occurs in some cases [33]. Aside from weakness, patients can develop autonomic dysfunction such as
arrhythmias (which in some cases necessitate pacemakers), blood pressure
lability, hyperhydrosis or ileus [34]. Pain, particularly severe back pain, is
also a commonly associated clinical feature [35], and in cases of bilateral
flaccid lower limb weakness, may complicate the differential as this could also
suggest the possibility of cauda equina syndrome or acute cord pathology - the
more prominent and persistent bladder and/or bowel disturbance with saddle
paraesthesia or sensory level, and confirmation with an urgent MRI spine, will
help distinguish these differential diagnoses. The diagnosis of GBS can be made using the Brighton criteria. This takes into
consideration the level of diagnostic certainty (graded from 1 to 4) for each
category of clinical examination findings (bilateral flaccid muscle weakness,
hypo- or areflexia, monophasic disease course from onset time to nadir),
ancillary investigations (CSF cell count <50/µl, raised CSF protein,
supportive nerve conduction study findings) and absence of an alternative
explanation for muscle weakness. Importantly, this has been validated in
several studies [32,36-38]. Classical
Miller-Fisher syndrome (MFS) (10%): Triad of ophthalmoplegia, areflexia
and ataxia associated with anti-GQ1b antibodies in 80-90% of cases [39]. Paraparetic GBS
(7%):
flaccid weakness of both lower limbs with relative sparing of other muscle
groups. Pharyngeal-cervical-brachial
subtype (5%):
weakness of bulbar, neck and upper limb muscles, and is associated with anti-GT1a
antibodies. This clinical syndrome may be misdiagnosed as myasthenia gravis or
botulism [7,40]. Bifacial weakness
with paraesthesia (3%):
the sensory disturbances (e.g. tingling) typically affect the distal
extremities. Bickerstaff
brainstem encephalitis (2%): MFS phenotype but with associated encephalopathy
and disrupted consciousness due to involvement of the ascending reticular
activating system [41,42]. Although
largely a clinical diagnosis, several ancillary investigations can be helpful
when confronted with a case of suspected GBS. Neurophysiology facilitates a
confident diagnosis, but also allows differentiation of the axonal (AMAN and
AMSAN) from demyelinating (AIDP) subtypes [14], which can assist in predicting
short and long-term prognoses [43-45]. The neurophysiological features of demyelinating variants include abnormal F
waves (which along with loss of the H reflex is amongst the earliest of
features within 1 week of muscle weakness), slowing of motor conduction
velocities, prolongation of distal motor latencies and temporal dispersion
[46]. Sparing of the sural Sensory Nerve Action Potential (SNAP) is
particularly characteristic of GBS. A significant reduction in the distal Compound
Muscle Action Potential (CMAP) amplitude (<80% of the lower limit of
normal), alongside the absence of demyelinating features, suggests axonal GBS
[14,47]. Electrophysiological abnormalities are evident in over 85% of patients
at least 2 weeks after the start of muscle weakness [48] and thus may be normal
early during its natural history. Thus, the diagnosis in the acute setting is
largely clinical. Analysis of Cerebrospinal Fluid (CSF) is helpful and classically reveals a
raised protein with normal cell count (albuminocytological dissociation), the
sensitivity of which is dependent on timing of lumbar puncture (raised CSF
protein is seen in 49% at day 1 and 88% after 2 weeks of weakness) [32]. CSF
white cell counts greater than 50 cells/μl would suggest an alternative
diagnosis [32] such as infective, inflammatory or neoplastic infiltration of
the brain, cord and/or meninges. Spinal MRI imaging is useful in excluding alternative differential diagnoses
that sometimes mimic classic GBS such as acute spinal disc prolapse, epidural
abscess/hematoma, cord infarction or transverse myelitis [7]. Nerve root
enhancement on gadolinium contrast MRIs can positively support a diagnosis of
GBS, and may provide useful information in electrophysiologically equivocal
cases [49].The diagnostic utility of testing serum for anti-ganglioside
antibodies can be of assistance such as for anti-GQ1b antibodies in MFS [50],
anti-GD1a and anti-GM1 for AMAN [51,52] and anti-GT1a for the PCB variant of
GBS [53]. However, the absence of anti-ganglioside antibodies does not exclude
the diagnosis for each GBS subtype. Due
to the heterogeneity of the disease, management is tailored to individual
patients and should be based on their pattern and severity of clinical
presentation. Various parameters must be closely monitored to identify those
individuals who are at risk of deterioration and require urgent supportive
care. Respiratory function should be observed and must include frequent checks
of the patients Forced Vital Capacity (FVC). As a generic rule, FVC values less
than 20ml/kg require escalation of care to the Intensive Care Unit (ICU) for
close monitoring and possibly endotracheal intubation – if the FVC is less than
15ml/kg, then this would require more serious consideration for prompt
intubation and mechanical ventilation [54]. Clinical models have been generated,
which allow for the prediction of risk of respiratory insufficiency and
subsequent requirement for mechanical ventilation within 1 week of symptom
onset [55]. Of note, pulse oximetry and arterial blood gas measurements are
inadequate for early detection of respiratory failure and they should not be
solely relied upon [56]. Haemodynamic monitoring of Blood Pressure (BP) and heart rate/rhythm is
imperative owing to risk of BP lability and cardiac autonomic disturbances,
which in severe cases, can lead to atrioventricular block or asystole
necessitating pacemaker insertion [57,58]. The cornerstone of therapy for GBS is Intravenous Immunoglobulin (IVIG) or Plasma
Exchange (PLEX) and their equivalent short and long-term benefits against
morbidity have been demonstrated in multiple Randomised-Controlled Trials
(RCTs) [60-63]. Oral corticosteroids or intravenous methylprednisolone are not
effective in hastening recovery or impacting long-term outcome in GBS [64]. IVIG
or PLEX should be commenced in patients with GBS who are unable to walk 10m
unaided (GBS disability scale score ≥ 3) at the earliest opportunity following
symptom onset [60]. IVIG hastens recovery from GBS as much as PLEX if given
within 2 weeks of symptom onset and RCTs have shown that IVIG is more likely to
be completed than PLEX, probably due to greater patient convenience (rates of
adverse events are equivalent overall in both groups) [62]. IVIG
is administered as a total dose of 2g/kg divided over 2 or 5 days, though it
remains unclear which duration, if any, is superior. Approximately 10% of
patients may clinically deteriorate following a period of stabilization after
their first treatment course of IVIG or PLEX, a phenomenon referred to as
Treatment-Related Fluctuation (TRF) [65]. Although, common practice involves commencing
a second course of the same treatment in such patient groups, the evidence for
this is sparse at the present time. Patients who continue to relapse after 8
weeks of symptom onset should have their diagnosis revised to acute-onset
Chronic Inflammatory Demyelinating Polyneuropathy (a-CIDP), which has long-term
therapeutic implications as these patients may require further courses of IVIG
and/or initiation of corticosteroids [66]. PLEX is beneficial if given within 4 weeks of symptom onset, but the effect
sizes are greater if given earlier, especially within 2 weeks [60-63]. It is
typically administered in 5 sessions over 2 weeks, with an exchange of 2-3L of
plasma per session, depending on body weight. The combination of PLEX followed
by IVIG is not superior to either treatment given alone [60-63]. The role of
IVIG or PLEX in mildly affected patients who remain ambulatory is unclear and
the evidence remains limited. As a pragmatic approach, and according to expert
opinion [67], treatment with IVIG/PLEX should be considered if such patients
also have significant autonomic dysfunction, bulbar or facial weakness [67].
Similarly, in patients with MFS, IVIG/PLEX should be given if there is
additional limb weakness during its course (MFS-GBS overlap), facial, bulbar or
respiratory weakness; otherwise in uncomplicated cases, supportive treatment
alone is often sufficient [67]. Despite
the aforementioned treatments, GBS has an overall estimated mortality of 3-12%
and up to one-fifth of survivors cannot walk unaided after 6 months [68,69].
Various prognostic models, such as the Erasmus GBS Outcome Scale (EGOS) and the
modified EGOS, have been generated and validated. These have shown that certain
clinical parameters, namely greater age (which is associated with greater
disability) [36,70], preceding diarrheal illness and a higher level of
disability within 1-2 weeks into the clinical course, are collaboratively
associated with a lower probability of independent ambulation at up to 6 months
[71,72]. Thus, these models can be used to predict which patients are more
likely to suffer from long-term residual disability, enabling more intensive
therapies, and future planning of supportive treatments, to be targeted to such
high-risk groups. However, it is important to note that residual disability is
not restricted to muscle weakness, but also encompasses fatigue, pain and
psychological morbidity, which can all impact on activities of daily living,
occupation and social functioning, and are not incorporated in these models. Conclusions GBS remains a significant worldwide cause of
rapidly progressive muscular paralysis. Although it is predominantly a clinical
diagnosis, neurophysiology, CSF analysis and neuroimaging are all helpful in
excluding potential mimics (and corroborating the diagnosis), which may
otherwise lead to diagnostic conundrums and therapeutic dilemmas. The majority
of studies that have assessed the role of therapies in GBS, namely IVIG and
PLEX, have been undertaken in North America and Europe, which have a higher
proportion of demyelinating GBS variants. The impact of these therapies
specifically in axonal GBS, which is more prevalent in certain Asian countries,
is less clear but nonetheless routinely recommended in clinical practice. Better
disease-modifying therapies are still required in GBS as a significant fraction
of patients suffer residual long-term neurological disability. References 1.
Guillain
G, Barré JA and Strohl A. On radiculo-neuritis syndrome with hyperalbuminosis
of cerebrospinal fluid without cellular reaction. Remarks on the clinical and
graphic characteristics of tendon reflexes (1916) Bull Soc Med Hop Paris
40:1462-1470. 2.
Sejvar
JJ, Baughman AL, Wise M and Morgan OW. Population incidence of Guillain-Barré
syndrome: a systematic review and meta-analysis (2011) Neuroepidemiology
36:123-133. 3.
Willison
HJ, Jacobs BC and Van Doorn PA. Guillain-Barré syndrome (2016) Lancet
388:717-727. 4.
McGrogan
A, Madle GC, Seaman HE and de Vries CS. The epidemiology of Guillain-Barré syndrome
worldwide. A systematic literature review (2009) Neuroepidemiology 32:150-163. 5.
Hiew
FL, Ramlan R, Viswanathan S and Puvanarajah S.
Guillain-Barré syndrome, variants and forms fruste: reclassification
with new criteria (2017) Clin Neurol Neurosurg 158:114-118. 6.
Naik
GS, Meena AK, Reddy BAK, Mridula RK, Jabeen SA et al. Anti-ganglioside
antibodies profile in Guillain-Barré syndrome: correlation with clinical
features, electrophysiology pattern, and outcome (2017) Neurol India
65:1001-1005. 7.
Wakerley
BR and Yuki N. Mimics and chameleons in Guillain-Barré and Miller Fisher
syndromes (2015) Pract Neurol 15:90-99. 8.
Winner
SJ and Evans JG. Age-specific incidence of Guillain-Barré syndrome in
Oxfordshire (1990) Q J Med 77:1297-1304. 9.
Jiang
GX, Cheng Q, Link H and de Pedro-Cuesta J. Epidemiological features of
Guillain-Barré syndrome in Sweden, 1978-93 (1997) J Neurol Neurosurg Psychiatry
62:447-53. 10.
Hughes
RA, Charlton J, Latinovic R and Gulliford MC. No association between
immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000
(2006) Arch Intern Med 166:1301-1304. 11.
Hankey
GJ. Guillain-Barré syndrome in Western Australia, 1980-1985 (1987) Med J Aust
146:130-133. 12.
Cheng
Q, Wang DS, Jiang GX, Han H, Zhang Y et al. Distinct pattern of age-specific
incidence of Guillain-Barré syndrome in Harbin, China (2002) J Neurol
249:25-32. 13.
Jiang
GX, de Pedro-Cuesta J and Fredrikson S. Guillain-Barré syndrome in south-west
Stockholm, 1973-1991, 1. Quality of registered hospital diagnoses and incidence
(1995) Acta Neurol Scand 91:109-117. 14.
Hadden
RD, Cornblath DR, Hughes RA, Zielasek J, Hartung HP, et al.
Electrophysiological classification of Guillain-Barré syndrome: clinical
associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome
Trial Group (1998) Ann Neurol 44:780-788. 15.
Doets
AY, Verboon C, van den Berg B, Harbo T, Cornblath DR, et al. Regional variation
of Guillain-Barré syndrome (2018) Brain 141:2866-2877. 16.
Ogawara
K, Kuwabara S, Mori M, Hattori T, Koga M, et al. Axonal Guillain-Barré syndrome:
relation to anti-ganglioside antibodies and Campylobacter jejuni infection in
Japan (2000) Ann Neurol 48:624-631. 17.
Kuwabara
S and Yuki N. Axonal Guillain-Barré syndrome: concepts and controversies (2013)
Lancet Neurol 12:1180-1188. 18.
Jacobs
BC, Rothbarth PH, van der Meché FG, Herbrink P, Schmitz PI, et al. The spectrum
of antecedent infections in Guillain-Barré syndrome: a case-control study
(1998) Neurology 51:1110-1115. 19.
Webb
AJ, Brain SA, Wood R, Rinaldi S and Turner MR. Seasonal variation in Guillain-Barré
syndrome: a systematic review, meta-analysis and Oxfordshire cohort study
(2015) J Neurol Neurosurg Psychiatry 86:1196-1201. 20.
Jackson
BR, Zegarra JA, Lopez-Gatell H, Sejvar J, Arzate F, et al. Binational outbreak
of Guillain-Barré syndrome associated with Campylobacter jejuni infection,
Mexico and USA, 2011 (2014) Epidemiol Infect 142:1089-1099. 21.
Salinas
JL, Walteros DM, Styczynski A, Garzon F, Quijada H, et al. Zika virus
disease-associated Guillain-Barré syndrome-Barranquilla, Colombia 2015-2016 (2017)
J Neurol Sci 381:272-277. 22.
Styczynski
AR, Malta JMAS, Krow-Lucal ER, Percio J, Nóbrega ME et al. Increased rates of
Guillain-Barré syndrome associated with Zika virus outbreak in Salvador
metropolitan area, Brazil (2017) PLoS Negl Trop Dis 11:1-13. 23.
Nyati
KK and Nyati R. Role of Campylobacter jejuni infection in the pathogenesis of
Guillain-Barré syndrome: an update (2013) Biomed Res Int 2013:1-13. 24.
Tam
CC, Rodrigues LC, Peterson I, Islam A, Hayward A et al. Incidence of
Guillain-Barré syndrome among patients with Campylobacter infection: a general
practice research database study (2006) J Infect Dis 194:95-97. 25.
Nachamkin
I, Allos BM and Ho T. Campylobacter species and Guillain-Barré syndrome (1998)
Clin Microbiol Rev 11:555-567. 26.
Halstead
SK, Zitman FM, Humphreys PD, Greenshields K, Verschuuren JJ, et al. Eculizumab
prevents anti-ganglioside antibody-mediated neuropathy in a murine model (2008)
Brain 131:1197-1208. 27.
Orlikowski
D, Prigent H, Sharshar T, Lofaso F and Raphael JC. Respiratory dysfunction in
Guillain-Barré syndrome (2004) Neurocrit Care 1:415-422. 28.
Fletcher
DD, Lawn ND, Wolter TD and Wijdicks EF. Long-term outcome in patients with
Guillain-Barré syndrome requiring mechanical ventilation (2000) Neurology
54:2311-2315. 29.
Dhar
R, Stitt L and Hahn AF. The morbidity and outcome of patients with
Guillain-Barré syndrome admitted to the intensive care unit (2008) J Neurol Sci
264:121-128. 30.
Rees
JH, Thompson RD, Smeeton NC and Hughes RA. Epidemiological study of Guillain-Barré syndrome in south east England
(1998) J Neurol Neurosurg Psychiatry 64:74-77. 31.
Winer
JB, Hughes RA and Osmond C. A prospective study of acute idiopathic neiropathy.
I. Clinical features and their prognostic value (1998) J Neurol Neurosurg
Psychiatry 51:605-612. 32.
Fokke
C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, et al. Diagnosis of
Guillain-Barré syndrome and validation of Brighton criteria (2014) Brain
137:33-43. 33.
Yuki
N, Kokubun N, Kuwabara S, Sekiguchi Y, Ito M, et al. Guillain-Barré syndrome
associated with normal or exaggerated tendon reflexes (2012) J Neurol
259:1181-1190. 34.
Zaeem
Z, Siddiqi ZA, Zochodne DW. Autonomic involvement in Guillain-Barré syndrome:
an update (2018) Clin Auton Res [Epub ahead of print]. 35.
Moulin
DE, Hagen N, Feasby TE, Amireh R and Hahn A. Pain in Guillain-Barré syndrome
(1997) Neurology 48:328-331. 36.
Al-Hakem
H, Sindrup SH, Anderson H, de la Cour CD et al. Guillain-Barré syndrome in
Denmark: a population-based study on epidemiology, diagnosis and clinical
severity (2018) J Neurol [Epub ahead of print]. 37.
Islam
MB, Islam Z, Farzana KS, Sarker SK, Endtz HP, et al. Guillain-Barré syndrome in
Bangladesh: validation of Brighton criteria (2016) J Periph Nerv Syst
21:345-351. 38.
Roodbol
J, de Wit MY, van den Berg B, Kahlmann V et al. Diagnosis of Guillain-Barré
syndrome in children and validation of the Brighton criteria (2017) J Neurol
264:856-861. 39.
Uchibori
A and Chiba A. Autoantibodies in Guillain-Barré syndrome (2015) Brain Nerve
67:1347-1357. 40.
Koga
M, Yuki N, Ariga T, Morimatsu M and Hirata K. Is IgG anti-GT1a antibody
associated with pharyngeal-cervical-brachial weakness or oropharyngeal palsy in
Guillain-Barré syndrome? (1998) J Neuroimmunol 86:74-79. 41.
Shahrizaila
N and Yuki N. Bickerstaff brainstem encephalitis and Fisher syndrome: anti-GQ1b
antibody syndrome (2013) J Neurol Neurosurg Psychiatry 84:576-583. 42.
Shameem
R, Sonpal N, Hamid M, Orsher S, Bhatia N, et al. Bickerstaffs brainstem
encephalitis: A rare variant of the Anti-GQ1b antibody syndrome (2013) Pract
Neurol pp:28-31 43.
Hiraga
A, Mori M, Ogawara K, Hattori T and Kuwabara S. Differences in patterns of
progression in demyelinating and axonal Guillain-Barré syndromes (2003)
Neurology 61:471-474. 44.
Hiraga
A, Mori M, Ogawara K, Kojima S, Kanesaka T, et al. Recovery patterns and long
term prognosis for axonal Guillain-Barré syndrome (2005) J Neurol Neurosurg
Psychiatry 76:719-722. 45.
Kalita
J, Kumar M and Misra UK. Prospective comparison of acute motor axonal
neuropathy and acute inflammatory polyradiculoneuropathy in 140 children with
Guillain-Barré syndrome in India (2018) Muscle Nerve 57:761-765. 46.
Gordon
PH and Wilbourn AJ. Early electrodiagnostic findings in Guillain-Barré syndrome
(2001) Arch Neurol 58:913-917. 47.
Van
den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, et al. Guillain-Barré
syndrome: pathogenesis, diagnosis, treatment and prognosis (2014) Nat Rev
Neurol 10:469-482. 48.
Albers
JW, Donofrio PD and McGonagle TK. Sequential electrodiagnostic abnormalities in
acute inflammatory demyelinating polyradiculoneuropathy (1985) Muscle Nerve
8:528-539. 49.
Gorson
KC, Ropper AH, Muriello MA and Blair R. Prospective evaluation of MRI
lumbosacral nerve root enhancement in acute Guillain-Barré syndrome (1996)
Neurology 47: 813-817. 50.
Chiba
A, Kusunoki S, Shimizu T and Kanazawa I. Serum IgG antibody to ganglioside GQ1b
is a possible marker of Miller Fisher syndrome (1992) Ann Neurol 31:677-679. 51.
Visser
LH, Van der Meché FG, Van Doorn PA, Meulstee J, Jacobs BC et al. Guillain-Barré
syndrome without sensory loss (acute motor neuropathy). A subgroup with
specific clinical, electrodiagnostic and laboratory features. Dutch
Guillain-Barré Study Group (1995) Brain 118:841-847. 52.
Yuki
N, Ho TW, Tagawa Y, Koga M, Li CY, et al. Autoantibodies to GM1b and
GalNAc-GD1a: relationship to Campylobacter jejuni infection and acute motor
axonal neuropathy in China (1999) J Neurol Sci 164:134-138. 53.
Wakerley
BR and Yuki N. Pharyngeal-cervical-brachial variant of Guillain-Barré syndrome
(2014) J Neurol Neurosurg Psychiatry 85:339-344 54.
Lawn
ND, Fletcher DD, Henderson RD, Wolter TD and Wijdicks EF. Anticipating
mechanical ventilation in Guillain-Barré syndrome (2001) Arch Neurol
58:893-898. 55.
Walgaard
C, Lingsma HF, Ruts L, Drenthen J, Koningsveld VR et al. Prediction of
respiratory insufficiency in Guillain-Barré syndrome (2010) Ann Neurol
67:781-787. 56.
Harms
M. Inpatient management of Guillain-Barré syndrome (2011) Neurohospitalist
1:78-84. 57.
Greenland
P and Griggs RC. Arrhythmic complications in the Guillain-Barré syndrome (1980)
Arch Intern Med 140:1053-1055. 58.
Patel
MB, Goyal SK, Punnam SR, Pandya K, Khetarpal V, et al. Guillain-Barré syndrome
with asystole requiring permanent pacemaker: a case report (2009) J Med Case
Rep 3:5. 59.
Bernsen
RA, de Jager AE, Schmitz PI and van der Meché FG. Long-term impact on work and
private life after Guillain-Barré syndrome (2002) J Neurol Sci 201:13-17 60.
Hughes
RA, Swan AV, Raphael JC, Annane D, Koningsveld VR, et al. Immunotherapy for
Guillain-Barré syndrome: a systematic review (2007) Brain 130:2245-2257. 61.
Raphael
JC, Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barré syndrome
(2012) Cochrane Database Syst Rev 7: CDD001798 62.
Hughes
RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré
syndrome (2014) Cochrane Database Syst Rev 9: CD002063 63.
Chevret S, Hughes RA, Annane D. Plasma
exchange for Guillain-Barré syndrome (2017) Cochrane Database Syst Rev 2:
CD001798 64.
Hughes
RA, Brassington R, Gunn AA, van DoornPA. Corticosteroids for Guillain-Barré
syndrome (2016) Cochrane Database Syst Rev 10:CD001446. 65.
Kleyweg
RP and van der Meché FG. Treatment related fluctuations in Guillain-Barré
syndrome after high-dose immunoglobulins or plasma-exchange (1991) J Neurol
Neurosurg Psychiatry 54:957-960. 66.
Ruts
L, Drenthen J, Jacobs BC, van Doorn PA and Dutch GBS Study Group.
Distinguishing acute-onset CIDP from fluctuating Guillain-Barré syndrome: A
prospective study (2010) Neurology 74:1680-1686. 67.
Verboon
C, van Doorn PA and Jacobs BC. Treatment dilemmas in Guillain-Barré syndrome
(2017) J Neurol Neurosurg Psychiatry 88:346-352. 68.
van
den Berg B, Bunschoten C, van Doorn PA and Jacobs BC. Mortality in
Guillain-Barré syndrome (2013) Neurology 80:1650-1654 69.
Netto
AB, Taly AB, Kulkarni GB, Rao UG and Rao S. Mortality in mechanically
ventilated patients of Guillain-Barré syndrome (2011) Ann Indian Acad Neurol
14:262-266. 70.
Peric
S, Berisavac I, Stojiljkovic TO, Rajic S, Babic M, et al. Guillain-Barré
syndrome in the elderly (2016) J Periph Nerv Syst 21:105-110. 71.
van
Koningsveld R, Steyerberg EW, Hughes RA, Swan AV, van Doorn PA, et al. A
clinical prognostic scoring system for Guillain-Barré syndrome (2007) Lancet
Neurol 6:589-594. 72.
Walgaard
C, Lingsma HF, Ruts L, van Doorn PA, Steyerberg EW, et al. Early recognition of
poor prognosis in Guillain-Barré syndrome (2011) Neurology 76:968-975. Corresponding author:Sanad Esmail, Norfolk
and Norwich University Hospitals, NHS Foundation Trust, Norwich,
UK, Tel: 01603 286286, E-mail: sanad.esmail2@gmail.com Citation: Esmail S. An
Overview of Guillain-Barré Syndrome (2019)
Neurophysio and Rehab 1: 42-46 GuillainBarré syndrome, Acute inflammatory
demyelinating polyneuropathy, Acute motor axonal neuropathy, Electrophysiology,
Intravenous immunoglobulin, Plasma exchangeAn Overview of Guillain-Barré Syndrome
Abstract
GuillainBarré Syndrome (GBS) is
an acute, autoimmune polyradiculoneuropathy that carries great patient
morbidity, and significant mortality, worldwide. The manifestations are highly
heterogeneous at the clinical, electrophysiological and biochemical levels,
which means that it is better to conceptualise GBS as a spectrum of disorders rather
than a singular one. Despite the diverse range of presentations, the management
of GBS is relatively stereotyped, albeit guided by the level of clinical
severity. Treatment is largely restricted to general supportive measures, Intravenous
Immunoglobulin (IVIG) and Plasma Exchange (PLEX), with no current role for oral
or intravenous corticosteroids in clinical practice. Several validated
prognosticscoring systems, which can predict the probability of longterm
residual disability, may assist in targeting intensive therapies to highrisk
patient groups. The aim of this article is to provide a practical overview of
GBS, with particular emphasis on the clinical presentation, investigation and
management of this important spectrum of neurological conditions.
Full-Text
Introduction
Epidemiology
Aetiology
Clinical Presentation
Other subtypes of GBS are recognised [5,7], which include:Investigations
Management
Deep vein thrombosis prophylaxis with low molecular weight heparin should be
considered in patients if there are no contraindications, pain should be
treated with analgesics and bladder and/or bowel dysfunction should be managed
appropriately. Physiotherapy input to facilitate mobilization, and to prevent
muscle deconditioning, alongside psychosocial support to help manage any
concomitant symptoms of depression or anxiety are also both crucial aspects of
supportive care [59]. Prognosis
Keywords