Research Article :
Objective:
To
determine what pattern of stroke lesions is responsible for VasP, as compared
to those patients who had stroke, gait and balance problems, but absence of a
hypokinetic rigid syndrome also called Vascular Pseudo Parkinsonism-V PSeuP. Materials
and Methods: Design: prospective cohort study. Participants
were consecutively screened for parkinsonian symptoms during a year as according
to our previous study. Validated questionnaire (Tanner Questionnaire-TQ) was
used, and a new scale operationalizing the criteria for VasP (FMAS score). All
participants in the original study had a clinical exam to identify if presence
of a hypokinetic rigid syndrome. Lesion patterns were analyzed. Setting: tertiary
care stroke prevention clinic at the University of Alberta Hospital. Participants:
Eligible participants attained a score of ≥ 4 on the TQ, high risk for
parkinsonism. Four groups were considered: V PseuP, VasP (onset of parkinsonism
within a year of the stroke -FMAS score of 4), Pseudo Vascular Parkinsonism-PseuVP
(hypokinetic rigid syndrome not related to stroke), and Pseudo Vascular Pseudo
Parkinsonism-PseuV PseuP (no stroke and no extrapyramidal syndrome), but with
gait and balance problems. Baseline demographic information and clinical
characteristics were recorded including vascular risk factors, and stroke
subtype. All participants had a Holter, CT head and/or brain MRI, and CTA. Medications
that produce drug-induced-parkinsonism were recorded for every participant. The
primary outcome was to find the pattern of anatomical lesions particularly
involved in the VasP subgroup considering the Basal Ganglia Motor Output
Circuit-BGMO, the Thalamo Cortical Drive Loop-TCD and connections to frontal
cortex. Results:
240 consecutive participants were screened during 12 months. We found 46
patients with potential Parkinsonism (TQ>4). VPseuP was found in 25/46
(54%), VasP in 8/46 (17%), PseuVP in 7/46 (15%), and PseuV PseuP in 6/46 (14%).
VasP were older (p<0.0007) and had a higher risk for cardio embolism due to
atrial fibrillation (p=0.02, odd ratio 6.6 CI 95% (1.2 – 35.2)). Neuro images
showed that only the pattern involving the BGMO and frontal cortex was
significantly associated to the group of VasP (X2 Fisher exact test p<0.0005
Odds ratio 32 CI 95% (9.6-108)); whereas the pattern TCD was not significantly
different between the groups (X2 Fisher exact test p=0.828 Odds ratio 1.2 CI
95% (0.5-2.8)). Discussion
and Conclusion: A two strategic location hit within the
BGMO circuit and frontal cortex is required, so a phenotype of VasP may occur. Critchley
described an “atherosclerotic parkinsonism” in patients with multiple strokes,
gait and balance problems and cognitive decline [1]. Subsequently Yamanouchi
described diffuse white matter lesions in the frontal lobes [2]. On 1989
FitzGerald and Jankovic utilized the term “lower body parkinsonism” noticing
that the parkinsonian feature was more prominent in the lower limbs [3]. Zijlman and colleagues [4] identified based on autopsies pathological lesions
that increased the BasalGanglia Motor Output (BGMO) including substantia nigra and lenticular
nucleus, and lesions that decreased the Thalamo Cortical Drive (TCD) involving
the ventro lateral area of the thalamus and frontal lobes. Recently, Viscarra
and colleagues have arguments against the previously defined syndrome,
referring to the low probability that strokes may present as true Parkinsonism,
and proposed 3 types of phenotypes. These phenotypes may differ according to
presence or not of Parkinsonism on the clinical exam, and presence or absence
of stroke. All these patients may present with gait and balance problems. Additionally,
they schematized the clinical manifestations and differentiated the affected
brain areas with patterns [5]. We
had the hypothesis that the pattern of ischemic lesions and network might be
different between those participants with Parkinsonism onset within 1 year from
stroke(s) (VasP) versus those with stroke and gait and balance problems, but
who never developed a hypokinetic rigid syndrome (V PseuP). Materials and
Methods This
study follows up our previous group of patients with stroke screened in a
tertiary care stroke prevention clinic in Alberta. All participants in the
original study had a clinical exam to identify if presence of a hypokinetic
rigid syndrome. Further details about that first study may be found elsewhere [6].
We selected from that database those patients at high risk for Parkinsonism
defined as those with high TannerQuestionnaire-TQ score (a screening validated questionnaire for the
identification of participants with Parkinsonism) that had a cut off score of ≥
4 which gave the highest sensitivity and specificity of this test, as confirmed
in ours and other previous studies
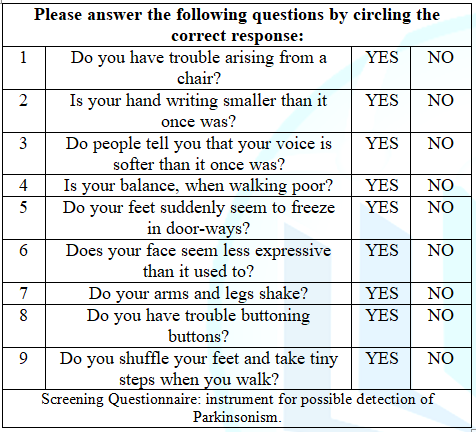
(Table 1). Table1: Tanner
Questionnaire: Screening Questionnaire. All participants were screened in our original study with a new scale
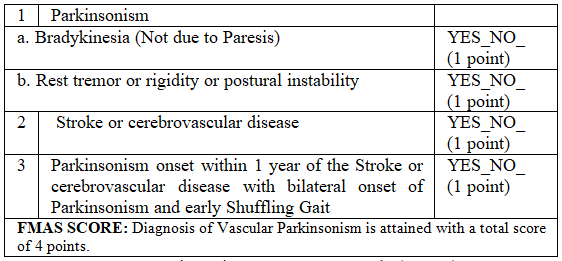
operationalizing the most recent criteria for Vascular
Parkinsonism the FMAS score [6] (Table
2). It consisted of item 1 and 2 corresponding to clinical criteria for
diagnosing and hypokinetic rigid syndrome, item 3 which considered a stroke(s)
confirmed by neuroimaging in the locations and network proposed by Zijlman to
be associated with onset of VasP. Item 4 correlated in time the onset of
Parkinsonism with the occurrence of the stroke(s) symptoms. A score of 2 would
be able to identify participants with Parkinsonism on clinical exam, and a
score of 4 was necessary to consider a clinical diagnosis of VasP [6]. Table 2: Five Minute
Assessment Scale (FMAS). The selection criteria for the prospective cohort of participants in this study
were patients attaining a TQ score ≥ 4 from our previous original study. We
classified the participants in four groups, according to presence or absence of
Parkinsonism on clinical exam, and presence of stroke and relationship between
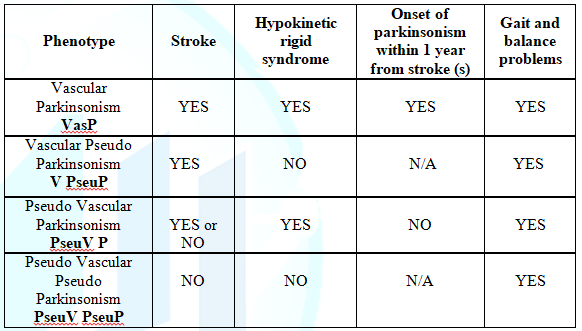
the onset of Parkinsonism and the occurrence of the stroke (Table 3). First subgroup: Pseudo Vascular Parkinsonism (PseuV P)
in which patients may have parkinsonism,
but there is no evidence of stroke in the neuroimaging or the location of the
stroke and timing of the lesion does not explain the clinical picture of parkinsonism;
the second subgroup or phenotype is Vascular Pseudo Parkinsonism (V PseuP) in
which patients may have acute symptoms related to stroke and the ischemic
lesion is confirmed by neuroimaging. These participants may present with gait
and balance problems, but on clinical exam there is no evidence of a
hypokinetic rigid syndrome. The third phenotype is PseudoVascular Pseudo Parkinsonism (PseuV PseuP) in which patients neither have
acute symptoms related to stroke and no cerebrovascular event is seen on
neuroimaging, or do they have and hypokinetic rigid syndrome on the exam. However,
these patients may have gait and balance problems. Finally the subgroup with
VasP in which the onset of Parkinsonism was present within 1 year from the
stroke(s) occurrence. Table 3: Different phenotypes
found in stroke patients with gait and balance problems TQ ≥ 4. Pseudo Vascular Parkinsonism (PseuVP) in which patients may have parkinsonism,
but there is no evidence of stroke in the neuroimaging or the location of the
stroke and timing of the lesion does not explain the clinical picture of
parkinsonism; the second phenotype is Vascular Pseudo
Parkinsonism (VPseuP) in which patients may have acute symptoms related to
stroke and confirmed by neuroimaging with gait and balance problems, but on
exam there is no evidence of a hypokinetic rigid syndrome. The third phenotype
is Pseudo Vascular Pseudo Parkinsonism (PseuVPseuP) in which patients neither
have acute symptoms related to stroke and no
cerebrovascular event is seen on neuroimaging, or do they have and hypokinetic
rigid syndrome on the exam. However, these patients may have gait and balance
problems. The study was approved by the Human Research Ethics Committee of the University
of Alberta, and informed consent was obtained from all participants attending
to the stroke prevention clinic. Demographic information was obtained from all participants, and the subtype of
stroke was assigned according to the TOAST classification [7] (Table 4). All participants had a
Holter, echo, CT head or brain MRI, and CTA. Neuroimaging
were reviewed by a senior fellow in movement disorders. Images were reported
initially by neuroradiology Table 4: Subtypes of
ischemic stroke-TOAST classification. Sample size was calculated based on prevalence of Vascular Parkinsonism found
previously in stroke care centers, and using the formula n=Z2 P
(1-P)/d2 [8]. Statistical analysis was done with the program SAS
software (copyright the SAS institute, Cary, N.C.), including in the second
study chi square, Fishers exact test for categorical variables, p value less
than 0.05 was consider of statistical significance, confidence intervals and
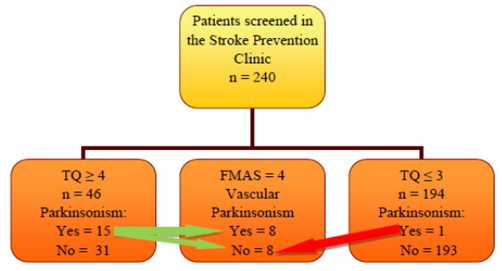
odds ratio were calculated too. Figure 1: Flow Diagram. Patients
enrollment.
Abbreviations: FMAS-Five-Minute Assessment Scale; TQ-Tanner Questionnaire. Modified
with permission from the copyright holder, Elsevier Journals. The source has
been acknowledged. From
240 patients screened, 16 were found to have Parkinsonism attaining at least a
score of 2 in the FMAS, and 15 of them with TQ ≥ 4 (Figure 1). The total group of TQ ≥ 4 was composed of 46 people. Demographic
data is shown in (Table 5). Patients
with VasP were older (p<0.0007), and had a higher risk for cardio embolism
(odds ratio 8.5, 95% CI (1.5-47.9), p=0.01) due to atrial fibrillation (odds ratio
6.6, 95% CI (1.2-35.2), p=0.02). We
described in detail the different phenotypes found within the group weather
they had an extrapyramidal syndrome, stroke syndrome, both or none accordingly (Figure 2). More than half of the group
(54%) had gait
and balance disturbances due to stroke, but no extra pyramidal For Table 5 click below We
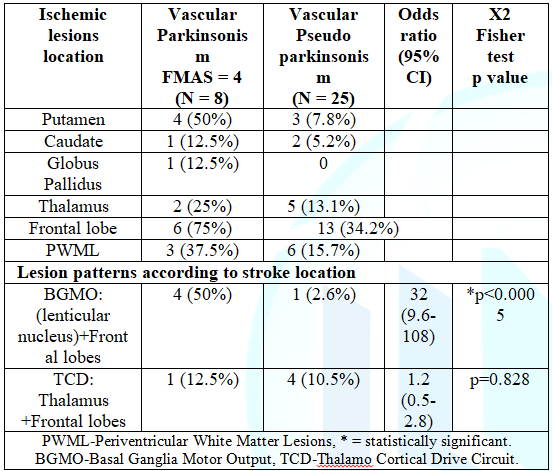
analyzed the pattern of ischemic brain lesions found in all participants (Table 6). We analyzed ischemic lesion
located at the BGMO and the TCD, and analyzed the most frequent patterns found.
The pattern involving the Lenticular nucleus (BGMO) and frontal lobes was
significantly associated to the group of VasP (X2 Fisher exact test p<0.0005,
odds ratio 32, 95% CI (9.6-108)); whereas the pattern Thalamus-Frontal Lobes
(TCD) was not significantly different between the two groups (X2 Fisher
exact test p=0.828, odds ratio 1.2, 95% CI (0.5-2.8)). The
prevalence of VasP in a tertiary care stroke prevention clinic was of 3%,
similar to what has been reported in other European, and American studies [9]. Other
groups have made the observation that VasP patients are older than patients
with idiopathic Parkinson
disease when comparing the onset of the extra pyramidal syndrome [10]. In
our cohort
we found similar results, participants with VasP were older than patients with
Parkinsonism due to other cause, or those who had a stroke(s) without an
extrapyramidal syndrome. Also, the high prevalence of A. Fib and cardio
embolism was related to the older age in this group of VasP. According to the
Framingham study, there is an exponential increase in the prevalence of A. Fib.
With aging our patients may also have decreased brain plasticity due to aging
[11,12]. We
propose a double hit theory in which the network that increase the basal
ganglia motor output is damaged from the lenticular nucleus substantia nigra
to the cortical frontal connections. Having a decreased output from the Globus
Pallidus externus (Lenticular nucleus) to the Subthalamic
Nucleus (STN), may preferentially favored the excitatory neurotransmitter
effect from the STN over the Globus Pallidus Internus and Substantia nigra pars
reticulate, consequently favoring the inhibitory output towards the thalamus
& cortex loop. This would be expressed clinically as limited movement with
bradykinesia [13].
Some of the weakness is that our previous study was a cross sectional study, so
new onset of a hypokinetic rigid syndrome could not be identified
prospectively. On the other hand, participants with lower TQ scores<4 were
unlikely to have Parkinsonism (1out of 193 participants) as data from our
previous investigation.
Future neuroimaging studies including Dopamine receptors/transporters and neuroimmune
modulatory molecules involved in this network are required to confirm our
findings: a double location hit within the BGMO and Lenticular nucleus, and the
frontal cortex, so a phenotype of VasP may occur. Funding The
original study on which this article is based was funded through a grant from
the Toupin foundation at the University of Alberta. Permission TQ
permission to use was obtained from the copyright holder, Wiley Co. The source
has been acknowledged. FMAS permission to use was obtained from the copyright
holder, Elsevier Journals. The source has been acknowledged.
1.
Critchley
M. Arteriosclerotic Parkinsonism (1929) Brain 52: 23-83. https://doi.org/10.1093/brain/52.1.23
2.
Yamanouchi
H and Nagura H. Neurological signs and frontalwhite matter lesions in vascular Critchley
Parkinsonism (1997) Stroke 28: 965-969. https://doi.org/10.1161/01.STR.28.5.965
3.
FitzGerald
P and Jankovic J. Lower body parkinsonism: evidence for vascular etiology
(1989) Mov Disord 4: 249-260. https://doi.org/10.1002/mds.870040306
4.
Zijlmans
JC, Daniel SE, Hughes AJ, Révész T and Lees AJ. Clinicopathological investigation
of Vascular Parkinsonism, including clinical criteria for diagnosis (2004) Mov
Disord 19: 630-640. https://doi.org/10.1002/mds.20083
5.
Vizcarra
JA, Lang AE, Sethi KD and Espay AJ. Vascular Parkinsonism: deconstructing a
syndrome (2015) Mov Disord 30: 886-894. https://doi.org/10.1002/mds.26263 6.
Manosalva
HA, Pio F, Jeerakathil T, Saqqur M, Camicioli R, et al. Vascular parkinsonism
in a tertiary care stroke prevention clinic and the development of a new
screening strategy (2018) J Stroke Cerebrovas dis 27: 153-161. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.08.020
7.
Adams
HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, et al. Classification of
subtype of acute ischemic stroke: definitions for use in a multicenter clinical
trial. Stroke (1993) 24: 35-41. 8.
Naing
L, Winn T and Rusli B. Practical Issues in Calculating the Sample Size for
Prevalence Studies (2006) Archives of Orofacial Sciences 1: 9-14. 9.
Foltynie
T. Vascular Parkinsonism: a review of the precision and frequency of the
diagnosis (2002) Neuroepidemiology 21:1-7. https://doi.org/10.1159/000048607 10.
Benítez-Rivero
S, Marín-Oyaga VA, García-Solís D, Huertas-Fernández I, García-Gómez FJ, et al.
Clinical features and 123I-FP-CIT SPECT imaging in vascular parkinsonism and
parkinsons disease (2013) J Neurol Neurosurg Psychiatr 84: 122-129. https://doi.org/10.1136/jnnp-2012-302618
11.
Wolf P, Abbott R and Kannel W. Atrial
fibrillation as an independent risk factor for stroke: the Framingham Study
(1991) Stroke 22: 983-988. 12.
Moratalla
R, Solis O and Suarez L. Handbook of Basal Ganglia structure and function, 2nd
edition (2016) Academic Press, USA 755-770. 13.
Park
J. Movement Disorders following cerebrovascular lesion in the Basal Ganglia Circuit
(2016) J Mov Dis 9: 71-79. https://dx.doi.org/10.14802%2Fjmd.16005
14.
Ghika
J, Bogousslavsky J and Regli F. Infarcts in the territory of lenticulostriate
branches from the middle cerebral artery. Etiological factors and clinical
features in 65 cases (1991) Schweiz Arch Neurol Psychiatr 142: 5-18. 15.
Korczyn A. Vascular parkinsonism-characteristics,
pathogenesis and treatment (2015) Nat Rev Neurol 11: 319-326. https://doi.org/10.1038/nrneurol.2015.61
16.
Mayasi
Y, Helenius J, McManus D, Goddeau J, Jun-OConnell A, et al. Atrial fibrillation
is associated with anterior predominant white matter lesions in patients
presenting with embolic stroke (2018) J Neurol Neurosurg Psychiatry 89 : 6-13. https://doi.org/10.1136/jnnp-2016-315457
Herbert
Alejandro Manosalva, Division
of Neurology, Department of Medicine, Sunnybrook Hospital-University of
Toronto, Canada. Tel: (647) 461-9044, Fax: 416-480-5753, E-mail:guiamesr@yahoo.co.uk Manosalva
HA. Double hit theory for the development of Vascular Parkinsonism (2019)
Neurophysio and Rehab 2: 42-46 Pathophysiology, Vascular Parkinsonism, Vascular
pseudo parkinsonism, Gait and balance problems, Neuroimaging, Basal ganglia
network in Parkinsonian disorders.Double Hit Theory for the Development of Vascular Parkinsonism
Herbert Alejandro
Manosalva
Abstract
Introduction:
Identify the non-decoded network in Vascular Parkinsonism (VasP). Full-Text
Introduction
y unaware of the different subgroups in the study. Data was extracted from our
previous study [6]. Stroke lesions were topographically identified, and
patterns were analyzed including the BGMO, the TCD, and frontal cortex.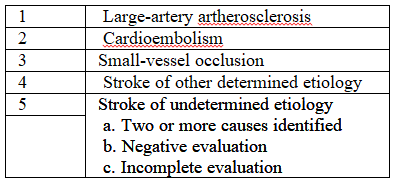
Results
syndrome was found on them (sub group VPseuP). It was followed by the patients
with VasP (17%), then PseuV P (15%) and the different diagnosis found in this
group, and finally PseuV PseuP (14%). 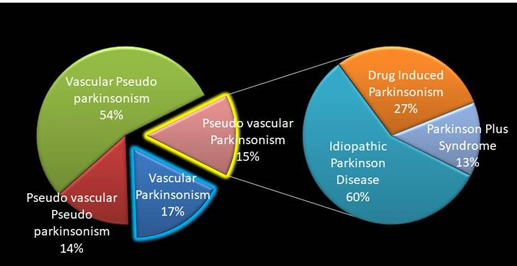
Table 5: Demographic
Characteristics of Participants with TQ ≥ 4 with and without Vascular
Parkinsonism.Discussion
Perforant arteries typically perfuse the deep structure of the basal ganglia
(lenticulo striate arteries), and frequently hypertension and diabetes mellitus
are the underlying risk factors. On the other hand, cortical strokes including
frontal lobes are frequently involved in cardio embolism, and in a smaller
percentage may also affect the deep structures of the basal ganglia too [14]. It
is important to point out that the most frequent vascular risk factors in our
cohort of patients with VasP were Diabetes
Mellitus and Atrial Fibrillation. Our study suggested the need for a double
hit ischemic injury at these brain locations (deep basal ganglia structures,
and frontal cortex), so consequently the phenotype of an extrapyramidal
syndrome may appear. The yield of involving both locations may become higher
when combining different mechanisms. Lipohyalinosis related to small vessel
occlusion applicable to the first location, also reported by other
investigators in VasP [15], and cardio embolism particularly due to A. Fib in
elderly population involving the frontal lobe applicable to the second location
[16].
Interestingly, we found that when the pattern of ischemic lesion involved the
frontal lobe and thalamus connections, participants had gait and balance
problems, but no Parkinsonism. References
*Corresponding author:
Citation:
Keywords