Introduction
Polyols have been used for decades as a substitute for
sucrose [1-4]. The most commonly used polyols for consumption are sorbitol,
mannitol, xylitol, erythritol, maltitol, lactitol and isomalt [5]. Besides
having fewer calories than regular sucrose, i.e., table sugar, polyols have
other reported health benefits, especially in regards to oral health [6]. Well
publicized studies showing the effectiveness of xylitol at reducing dental
disease have been reported for decades, all with results demonstrating safety
and effectiveness [7-9]. The well-publicized “Turku” and the “Belize” studies
reported on the caries reduction by xylitol, with xylitol being more effective
than sorbitol [10,11]. Xylitol chewing gums, toothpastes, lollipops, candies
and mouth rinses are all part of a complete dental oral hygiene program [12]. Erythritol
and xylitol are polyols that repeatedly have been demonstrated to possess
anti-cariogenic and anti-periodontal disease properties [13]. Polyols
(particularly the non-hexitol alditols or “sugar alcohols” erythritol and
xylitol) have been found effective in inhibiting the transition to and
maturation of biofilms from planktonic cells [14]. Xylitol clearly inhibits the
formation of mixed species biofilms, in vitro [15].
Erythritol suppresses the maturation of biofilms and
contributed to a healthier oral ecosystem [16]. Polyols can suppress the growth
and virulence expression of mixed bacterial biofilms. Erythritol was the most
effective polyol in suppressing the growth and organization of dental
pathogens. Erythritol also exerted inhibitory effects on several pathways reduced
growths through DNA and RNA depletion, attenuated extracellular matrix
production and alterations of dipeptide acquisition and amino acid metabolism
[17].
The bacteria associated with Autism Spectrum Disorder have
been reported in the literature, with similar results independent of research
institution and locality [18]. Autism Spectrum Disorder (ASD) has been linked
to propionic acid producing bacterial species, such as, Clostridia bolteae and Clostridia
histolyticum [19-22]. Conversely the presence of Clostridia sporogenes
could help protect against ASD by combining propionic acid with indole to
produce 3-Indole Propionate, a neural protective metabolite, thereby
neutralizing the epigenetic effect of propionic acid [23-25]. It has been
theorized that the absence of C.
sporogenes in the soil is related to the use of glyphosate, known by the
trade name Roundup [18]. Absence of C.
sporogenes in the soil and the environment could possibly shift the
maternal microbiome, resulting in epigenetic changes in the fetus or infant.
Bacteroides vulgatus also has been implicated in ASD as reported in the
Frontiers in Microbiology by Coretti et al. [26].
Clostridia difficile (Cdif) is a gram-positive bacterium
that is implicated in antibiotic-associated diarrhea. The relatively recent
emergence of a newer hyper-virulent North American strain (NAP1) has been
associated with the increase in incidence and severity of C. difficile infections (CDI) over the last decade [27]. Antibiotic
overuse remains the leading risk factor for C.
difficile infection. Several classes of antibiotics such as penicillins,
cephalosporins, fluoroquinolones, and clindamycin have been implicated in
causing CDI.
Besides antibiotic usage, other risk factors are reported to
include advanced age, chemotherapy, use of proton pump inhibitors, chronic
renal disease, chronic liver disease and malnutrition [28,29]. Treatment
options include discontinuing the causative antibiotic and administering either
vancomycin or fidaxomicin. Another option is fecal transplantation, the process
in which feces from a healthy donor are transplanted into the intestinal tract
of a person with the disrupted microbial balance. This protocol has reported an
80% to 90% success rate in reducing the recurrence of C. difficile infections [30]. There remains some opposition to
Fecal Transplantation Therapy due to the basic nature of the procedure and
potential complications [31]. A simpler, safer and “cleaner” technique would be
more appealing to patients and clinicians.
Materials and Methods
Bacterial isolates and media: C. bolteae
and C. histolytica strains were
kindly provided by Dr. Emma Allen- Verco PhD. (University of Guelph/Canada). B. vulgatis (8482) and B. longum (15707) were obtained from the
American Type Culture Collection (ATCCC/Manassas Va.). C. difficile strains 5555 and 5557 were provided by Dr. Larry
Kociolek MD (Lurie Children’s Hospital, Chicago, IL). All studies used a basal
media of Brain Heart Infusion broth supplemented with 2% sucrose (BHI/Suc).
Polyols were prepared separately at high concentrations in BHI/Suc for assay
plate preparations. Xylitol was added to 60% (w/v) and Erythritol was prepared
at 30% (w/v) in BHI/Suc. These polyol levels were the maximum achievable based
on solubility. Final media preparations were sterilized and placed in an
anaerobic chamber for at least 2 hours after preparation to cool and remain in
a reduced state.
Assay Procedures
Assays were prepared in the anaerobic chamber. 96 well
plates were employed with each test preparation in triplicate wells by adding
100 mcL of BHI/Suc at 2x concentration to all test wells. Bacterial
preparations were made in BHI/Suc adjusted to a Macfarland standard
concentration of 0.5. Final assay inocula of each strain with a further 1:100
fold dilution. 100 mcL of bacterial inocula was added to each test well with or
without a polyol. Plates were incubated anaerobically for 24 or 48 hours and
terminated when bacterial growth reached a easily visible level in control
wells. Plate were then transferred to a plate spectrophotometer and read at 620
nm wavelength. Mean OD values for each well were calculated and OD values vs.
polyol concentration were plotted.
Results
Seven strains were tested for polyol inhibitory activity C. histolyticum, B. vulgatus, C. bolteae
(x2), C. difficile (x2), and Bifidobacterium longham. All strains
grew to variable bacterial density levels. B. vulgatus had the poorest growth
but still had measurable mean OD values to suggest polyol activity. Detailed OD
values vs. polyol concentration are plotted as follows with relative inhibition
inflection points (Figures 1-7).
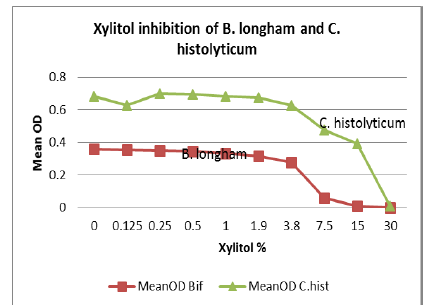
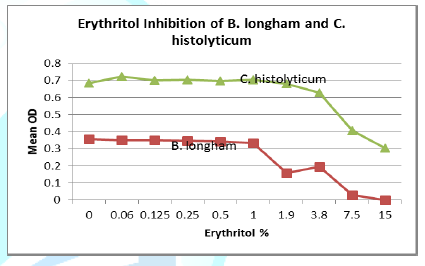
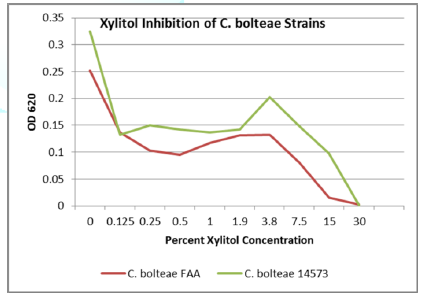
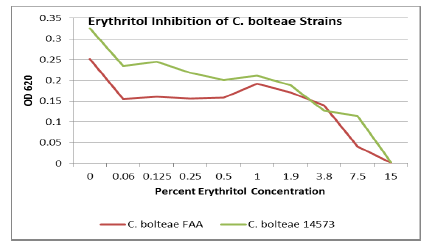
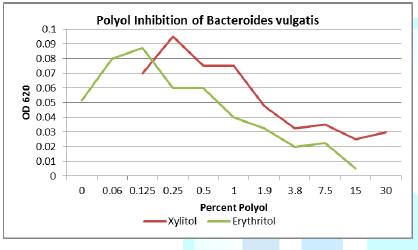
Figure 5: Both erythritol and xylitol inhibits B. vulgatis at only a 0.25% concentration.
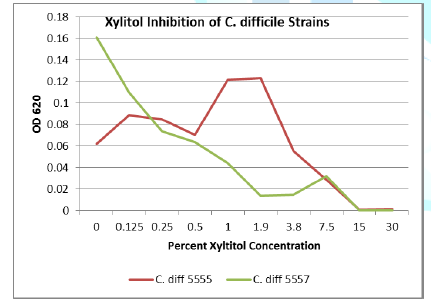
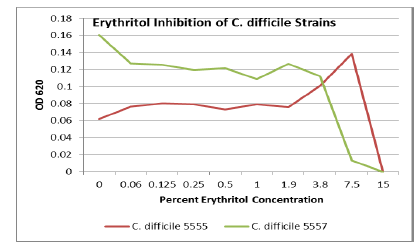
Discussion
Erythritol inhibits ASD bacteria at a lower concentration
than xylitol. Both polyols were capable of significant inhibition of the ASD
associated bacteria, in addition to the inhibition of antibiotic resistant C. diff strains. Erythritol may inhibit Bacteroides vulgatus better than xylitol
but additional studies with a more optimal media for B. vulgatis need to be performed. However, Xylitol should be
considered as a treatment for C.
difficile infection due to its low cost and availability. In addition,
xylitol and erythritol are considered safe food additives with decades of use
in the prevention of oral diseases, such as periodontal disease and dental
cavities.
Autism spectrum disorders are likely caused by a combination
of microbiome, environment, and the epigenetic interaction [32-34]. Recent
research shows that more than 50% of children with autism have GI symptoms,
food allergies, and maldigestion or malabsorption issues [35]. Propionic acid
is used as a food additive and is also a bacterial byproduct. Propionic acid
uptake may be related to lack of the bacterial gluten metabolizers and
resultant leaky gut. Elimination of calcium propionate as a bread
additive/preservative may be beneficial in reducing the behaviors associated
with ASD [36]. Shifting the oral and gut microbiome with polyols may also be
successful in reducing behaviors associated with ASD. More research, large
well-designed clinical trials are indicated for protocols illuminating
therapies effective with reducing the symptoms of ASD [37].
Conclusion
Xylitol and erythritol at sufficient concentrations were
able to inhibit the growth of bacterial strains that have been associated with
the development of ASD. Further research into the use of polyols for the
treatment and possible prevention of ASD is recommended. Large clinical trials
with patients that are correctly diagnosed with ASD then treated with xylitol
supplementation and the resultant effects on behavior should be carefully
explored. In addition, the uses of polyols to treat C. difficile infections also require clinical trials.
References
- Horecker BL, Lang K, Takagi Y.
International symposium on metabolism, physiology and clinical uses of pentoses
and pentitols (1969) Springer-Verlag, Berlin, Germany.
- Sipple HL, McNutt KW. Sugars in
Nutrition (1974) Academic Press, New York, USA.
- Hefferen JJ, Koehler HM. Foods,
Nutrition and Dental Health (1981) Pathotox Publishers, Park Forest South, IL,
USA.
- Rugg-Gunn J. Sugarless, the Way
Forward: Proceedings of an International Symposium (1991) Elsevier Applied
Science, London, UK.
- Rice T, Zannini E, Arendt EK, Coffey
A. A review of polyols - biotechnological production, food applications,
regulation, labeling and health effects (2019) Crit Rev Food Sci Nutr Pp: 1-18.
https://doi.org/10.1080/10408398.2019.1625859
- Scheie OB, Fejerskov. Xylitol in
caries prevention: what is the evidence for clinical efficacy? (1998) Oral Dis
4.
- Edgar WM. Sugar substitutes, chewing
gum and dental caries - a review (1998) British Dental Journal 184: 29-32.
- Mandel D. Caries prevention –
current strategies, new directions (1996) J American Dental Association 127:
1477-1488.
- Trahan L. Xylitol: a review of its
action on mutans streptococci and dental plaque - its clinical significance
(1995) The International Dental Journal 45: 77-92.
- Scheinin A, Mäkinen KK, Kalevi Y.
Turku sugar studies. V. Final report on the effect of sucrose, fructose and
xylitol diets on the caries incidence in man (1976) Acta odontologica Scandin
34: 179-216. https://doi.org/10.3109/00016357608997711
- Mäkinen KK, Bennett CA, Hujoel PP.
Xylitol chewing gums and caries rates: a 40-month cohort study (1995) J Dent
Res 74: 1904-1913. https://doi.org/10.1177/00220345950740121501
- Cannon ML and Peldyak JN. The
prevention and treatment of neural arterial gingival simplex (2019) Dental Res
Manag 3: 32-37. https://doi.org/10.33805/2572-6978.123
- Sánchez MC, Romero-Lastra P,
Ribeiro-Vidal H, Llama-Palacios A and Figueroa E. Comparative gene expression
analysis of planktonic Porphyromonas gingivalis ATCC 33277 in the presence of a
growing biofilm versus planktonic cells (2019) BMC Microbiol 19: 58. https://doi.org/10.1186/s12866-019-1423-9
- Badet C, Furiga A and Thébaud
N.Effect of xylitol on an in vitro model of oral biofilm (2008) Oral Health
Prev Dent 6: 337-341.
- Janus MM, Volgenant CMC, Brandt BW,
Buijs MJ, Keijser BJF, et al. Effect of erythritol on microbial ecology of in
vitro gingivitis biofilms (2017) J Oral Microbiol 9: 1. https://doi.org/10.1080/20002297.2017.1337477
- Hashino E, Kuboniwa M, Alghamdi SA,
Yamaguchi M, Yamamoto R, et al. Erythritol alters microstructure and
metabolomic profiles of biofilm composed of Streptococcus gordonii and
Porphyromonas gingivalis (2013) Mol Oral Microbiol 28: 435-451.
https://doi.org/10.1111/omi.12037
- Janakiram C, Deepan Kumar CV and
Joseph J. Xylitol in preventing dental caries: a systematic review and
meta-analyses (2017) J Nat Sci Biol Med 8: 16-21. https://doi.org/10.4103/0976-9668.198344
- Argou-Cardozo I and Zeidán-Chuliá F. Clostridium Bacteria and Autism Spectrum Conditions: A Systematic Review and Hypothetical Contribution of Environmental Glyphosate Levels (2018) Med Sci (Basel) 6: 29. https://doi.org/10.3390/medsci6020029
- MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders (2007) Behav Brain Res 176: 149-169. https://doi.org/10.1016/j.bbr.2006.07.025
- Shultz SR, MacFabe DF, Ossenkopp KP, Scratch S, Whelan J, et al. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism (2008) Neuropharmacology 54: 901-911. https://doi.org/10.1016/j.neuropharm.2008.01.013
- Shultz SR, Macfabe DF, Martin S,
Jackson J, Taylor R, et al. Intracerebroventricular injections of the enteric
bacterial metabolic product propionic acid impairs cognition and sensorimotor
ability in the Long-Evans rat: further development of a rodent model of autism
(2009) Behav Brain Res 200: 33-34. https://doi.org/10.1016/j.bbr.2008.12.023
- MacFabe DF, Cain NE, Boon F,
Ossenkopp KP and Cain DP. Effects of the enteric bacterial metabolic product
propionic acid on object-directed behavior, social behavior, cognition, and
neuroinflammation in adolescent rats: Relevance to autism spectrum disorder
(2011) Behav Brain Res 217: 47-54. https://doi.org/10.1016/j.bbr.2010.10.005
- Rose S, Bennuri SC, Davis JE, Wynne
R, Slattery JC, et al. Butyrate enhances mitochondrial function during
oxidative stress in cell lines from boys with autism (2018) Translational
Psychiatry 8: 42. https://doi.org/10.1038/s41398-017-0089-z
- Wikoff WR, Anfora AT, Liu J, Schultz
PG, Lesley SA, et al. Metabolomics analysis reveals large effects of gut
microflora on mammalian blood metabolites (2009) Proceedings of the National
Academy of Sciences of the United States of America, USA 106: 3698-3703. https://doi.org/10.1073/pnas.0812874106
- Parthasarathy A, Cross PJ, Dobson R, Adams LE, Savka MA, et al. A Three-Ring Circus: Metabolism of the three proteogenic aromatic amino acids and their role in the health of plants and animals (2018) Front Mol Biosci 5: 29. https://doi.org/10.3389/fmolb.2018.00029
- Coretti L, Paparo L, Riccio MP,
Amato F, Cuomo M, et al. Gut microbiota features in young children with autism
spectrum disorders (2018) Frontiers in microbiology 9: 3146.
https://doi.org/10.3389/fmicb.2018.03146
- See I, Mu Y, Cohen J, Beldavs ZG,
Winston LG, et al. NAP1 strain type predicts outcomes from Clostridium
difficile infection (2014) Clin Infect Dis 58: 1394-1400.
- Khanafer N, Vanhems P, Barbut F,
Luxemburger C, CDI01 Study group, et al. Factors associated with Clostridium
difficile infection: A nested case-control study in a three year prospective
cohort (2017) Anaerobe 44: 117-123.
- Arriola V, Tischendorf J, Musuuza J,
Barker A, Rozelle JW, et al. Assessing the risk of hospital-acquired
clostridium difficile infection with proton pump inhibitor use: a meta-analysis
(2016) Infect Control Hosp Epidemiol 37: 1408-1417.
- Kassam Z, Lee CH, Hunt RH. Review of
the emerging treatment of Clostridium difficile infection with fecal microbiota
transplantation and insights into future challenges (2014) Clin Lab Med 34:
787-798.
- van Beurden YH, de Groot PF, van
Nood E, Nieuwdorp M, Keller JJ, et al. Complications, effectiveness, and long
term follow-up of fecal microbiota transfer by nasoduodenal tube for treatment
of recurrent Clostridium difficile infection (2017) United European
gastroenterology journal 5: 868-879. https://doi.org/10.1177/2050640616678099
- Macfabe DF. The role of enteric
bacterial metabolites in mitochondrial dysfunction in autism – from animal
models to human population. Microb Ecol Health Dis.
- Midtvedt T. The gut: a triggering
place for autism – possibilities and challenges (2012) Microb Ecol Health Dis
23. https://doi.org/10.3402/mehd.v23i0.18982
- Mangiola F, Ianiro G, Franceschi F,
Fagiuoli S, Gasbarrini G, et al. Gut microbiota in autism and mood disorders
(2016) World J Gastroenterol 361-368. https://doi.org/10.3748/wjg.v22.i1.361
- Horvath K, Papadimitriou JC,
Rabsztyn A, Drachenberg C, Tildon JT. Gastrointestinal abnormalities in
children with autistic disorder (1999) J Pediatr 135: 559-563.
- Dengate S and Ruben A. Controlled trial of cumulative behavioural effects of a common bread preservative (2002) J Paediatrics and child health 38: 373-376. https://doi.org/10.1046/j.1440-1754.2002.00009.x
- Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, et al. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota (2019) Scientific reports 9: 5821. https://doi.org/10.1038/s41598-019-42183-0
*Corresponding author:
Cannon ML, Ann
and Robert Lurie Children’s Hospital, Northwestern University,Feinberg School of Medicine, Chicago, USA,
E-mail:
drmarkcannon@outlook.com
Citation:
Cannon
ML, Merchant M, Kabat W, Unruh B and Ramones A.
Inhibition
of autism spectrum disorder associated bacteria and c. difficile by polyols (2020) Edelweiss
Appli Sci Tech 4: 33-36.
Keywords
Autism Spectrum Disorder, Polyol, Bacterial strains,
Optical density.


 PDF
PDF