Research Article :
The advantages of albumin over less costly
alternative fluids continue to be debated. Many scientific articles were
devoted to the clinical analysis of the use of albumin in acute illness as well
as its comparison with other fluid regimens. However, the lack of fundamental
knowledge about the physical and chemical properties of commercial albumin
generates many unpromising discussions about the effectiveness of the use of
albumin among practitioners and medical scientists. The manuscript provides
information about the different variants of commercial albumin, the mechanisms of
their action, indications and contraindications to use. The main purpose of
this article is to objectively show the failure of generalizing conclusions and
recommendations on the clinical use of commercial albumin, taking into account
its various physical and chemical characteristics. To date, all studies should
be conducted either in the form of a comparative analysis of a specific
clinical effect, or within the framework of studies of only one brand of
albumin. Otherwise, generalizing the conclusions, the recommendations on the
use of different forms of albumin are not correct and generate a lot of useless
of the discussions. The presented information is based on fundamental knowledge
of physical and chemical properties of commercial albumin. This manuscript is
not only educational information, but also is scientific guide for clinicians. Background Human
Albumin (HA) or serum albumin is encoded by the ALB gene and is the most
abundant plasma protein in mammals. Human albumin is essential for maintaining
the osmotic pressure needed for proper distribution of body fluids between
intravascular compartments and body tissues. Human albumin also acts as a
plasma carrier by non-specifically binding several hydrophobic steroid hormones
and as a transport protein for hemin and fatty acids. The
advantages of albumin over less costly alternative fluids continue to be
debated. Meta-analyses focusing on
survival have been inconclusive, and other clinically relevant end-points have
not been systematically addressed. Database
searches (MEDLINE, EMBASE, Cochrane Library) and other methods were used to
identify randomized controlled trials comparing albumin with crystalloid,
artificial colloid, no albumin or lower-dose albumin. Major findings for all
end-points were extracted and summarized [1,2]. Seventy-nine randomized trials
with a total of 4755 patients were included. No significant treatment effects
were detectable in 20/79 (25%) trials. In cardiac surgery, albumin
administration resulted in lower fluid requirements, higher colloid oncotic
pressure, reduced pulmonary edema with respiratory impairment and greater
hemodilution compared with crystalloid and hydroxyethylstarch increased
postoperative bleeding. In non-cardiac surgery, fluid requirements, and
pulmonary and intestinal edema were decreased by albumin compared with
crystalloid. In hypoalbuminemia, higher doses of
albumin reduced morbidity. In ascites, albumin reduced hemodynamic
derangements, morbidity and length of stay and improved
survival after spontaneous bacterial peritonitis. In sepsis, albumin decreased
pulmonary edema and respiratory dysfunction compared with crystalloid, while
hydroxyethylstarch induced abnormalities of hemostasis. Complications were
lowered by albumin compared with crystalloid in burn patients.
Albumin-containing therapeutic regimens improved outcomes after brain injury
[3]. Neither benefit nor harm was shown when using HA to maintain hemodynamic
stability in the perioperative period when compared with crystalloids or any
other colloidal volume substitute [4, 5, 6]. In
a recent study, the effects of crystalloids and colloids, including HA, on
pulmonary edema in hypovolemic septic and non-septic patients with, or at risk
of, acute lung injury/acute respiratory distress syndrome were assessed. Pulmonary
edema and lung injury score were not affected by the type of fluid indicating
that HA was not superior to cheaper alternatives [7]. In
a cohort, multicentre, observational study of 3147 ICU patients, the use of HA
in European ICUs and its relationship to outcome were assessed [8]. The
indication for giving albumin was not specified (hypovolemia or
hypoalbuminemia). Three hundred and fifty-four patients (11.2%) received
albumin and 2793 patients (88.8%) did not. Albumin
administration
was associated with decreased survival in this population of acutely ill
patients. An international prospective cohort study including 1013 ICU patients
needing fluid resuscitation for shock documented that
the use of hyperoncotic albumin (20% HA) was significantly associated with
occurrence of negative renal events (two-fold increase in creatinine or need
for dialysis) and an increased risk of death in ICU [9]. International
guidelines for the management of severe sepsis and septic shock do not
specifically recommend the use of HA for volume replacement for hemodynamic
stabilization in this setting [10]. Shorter
hospital stay and lower costs were shown when using hyperoncotic HA for
correction of hypovolemia patients with
liver disease. Two studies, which were more than 15 year old, reported reduced
disability after using hyperoncotic albumin in brain injury. This review
conflicts with negative results from large studies showing increased mortality
after the use of HA in brain injury patients [11] and increased incidence of
renal failure in ICU patients after 20% HA [12]. Despite
these meta-analyses, it still remains unclear when to use HA. An evaluation in
53 hospitals in the USA showed that based on guidelines developed by the
University Health System Consortium (UHC), HA was inappropriately used for
57.8% of adult patients and 52.2% of pediatric patients [13,14]. It
is necessary to consider topical issues related to the fundamental knowledge
for the effective and adequate use of albumin drugs in clinical practice. Before
examine topic of methods, indications and contraindications the use albumin
solutions it is necessary determine: what type albumin we using? Is it native
or commercial types? What is their difference and is this knowledge important
for the medical practitioner? Native Albumin Albumins
(lat: albus, white) are simple water-soluble proteins. The molecule of albumin
is an ellipse of 3 × 15 nm, (3.6 Nm) that consists of a single polypeptide
chain. Its a low molecular weight protein. The relative molecular weight of
albumin is approximately 65000 Dt. Native albumin is synthesized in the liver
at a rate of about 10-15 mg per day. The 1 g of native albumin carries 20 ml of
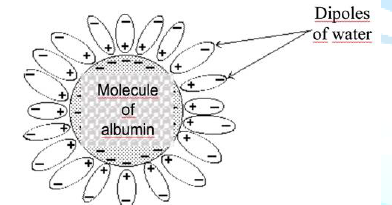
water into the vessels. The scheme of hydrophobic protein-ligand binding is
shown in Figure 1. Figure
1:
Protein-ligand hydrophobic binding. Figure
1 shows that one albumin molecule absorbs a large number of water dipoles. Commercial Albumin Commercial
albumin
is a stabilized and pasteurized native albumin. To date, there is a problem of
quantitative content of stabilizers and other additional substances in the
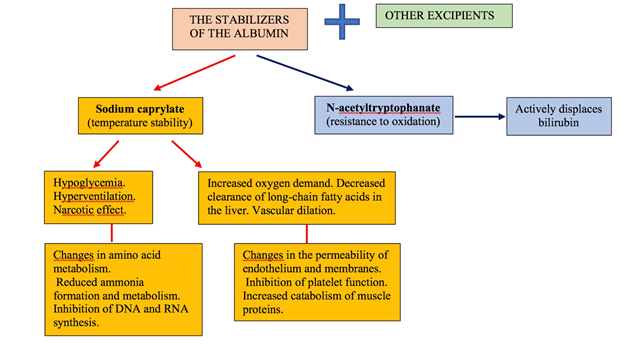
commercial preparation of albumin. The main stabilizers of albumin, their role
and influence on organism were presented in Figure 2 [15,16]. Figure 2 is shown that
composition of the commercial solution of albumin contains the various
preservatives and stabilizers. Among them, the main ones are
N-acetyltryptophanate and sodium caprylate. N-acetyltryptophanate to protect
the protein from oxidative stress and to stabilize it for heat treatment which
is applied for virus inactivation. In addition, it is important to say that
N-acetyltryptophan competes for active binding centers with bilirubin. Therefore,
the amount of N-acetyltryptophanate affects the sorption capacity of stabilized
albumin in relation to bilirubin. In the end this determines the efficacy
application of albumin with the aim of detoxication in hyperbilirubinemia. Sodium caprylate is the second
and mandatory preservative that provides temperature stability of albumin
molecules during pasteurization. Figure 2: The main
stabilizers of albumin, their role and influence. It
is very important to note that sodium caprylate causes a large number of
serious side effects, such as: hypoglycemia, hyperventilation, increases tissue
oxygen demand, prevents the metabolism of amino acids, inhibits the synthesis
of DNA and RNA, violates the permeability of the endothelium and membranes,
increases the catabolism of muscle proteins, inhibits platelet function, etc.
In connection with the toxicity of sodium caprylate control over the quality of
the Food and Drug Administration (FDA) considers necessary to carry the index
of its contents to the security settings of solutions of albumin. It
is also known that the binding capacity of commercial
albumin is significantly lower than that of native albumin due to the
congestion of its binding centers with stabilizers and other additional
substances. The lack of control over the content of lipids and fatty acids at
the stages of the technological process of albumin production affects its
binding capacity and transport function. Therefore,
as an additional indicator of the quality of albumin drugs, clinicians in many
countries propose to introduce into the regulatory documentation a quantitative
assessment of functional activity - binding capacity (binding capacity/sorption
capacity). Today
companies which produce commercial albumin in different countries do not have a
single approach to solving this problem. It follows from this that patients
receiving albumin drugs on the background of drug treatment are exposed to high
concentrations of free active substance. This naturally determines the risk of
exceeding pharmacological and side effects for the patient, which directly
threatens his life, and the detoxification function of commercial albumin is
doubtful. Also
necessary aware that some medications can compete for binding centers in albumin molecule with bilirubin
and among themselves. Only these facts are enough to not only take a critical
look at the results of the study of domestic and foreign authors, based on the
meta-analysis of the use of commercial albumin, but also with great caution to
follow their recommendations regarding the universality of its methods of
application, indications and contraindications. The
thoughtless and blind imposition by some domestic authors of
"universal" foreign recommendations for the use of commercial albumin
against the background of the lack of a single form of it is not only
scientifically doubtful, but also clinically unsafe for the patient. For
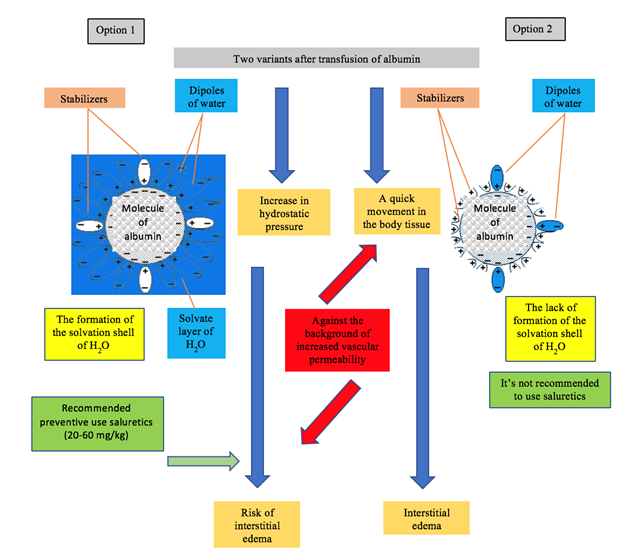
example, consider the action of two variants of commercial albumin (Figure 3). Figure 3: The scheme
action of the two options of commercial albumin. In
option 1 stabilizers block a small number of binding centers. In this case the
reserve binding capacity of albumin remains high enough. This variant of stable
albumin absorbs a significant amount of water. As a result, the hydrostatic
pressure in the vessels increases. Therefore,
to prevent the risk of interstitial
edema
(especially against the background of increased capillary permeability), after
transfusion of albumin, it is recommended to immediately administer diuretics
(saluretics at a dose of 20-60 mg/kg). This achieves anti-edema and
detoxification effects [17,18]. On
the contrary, in Option 2 stabilizers block a large number of binding centers.
Indicator the reserve binding capacity of albumin are low, and the sorption of
water molecules is insignificant. As a result, against the background of
increased capillary
permeability,
albumin quickly leaves the vascular bed and passes into interstitial tissues,
causing or increasing edema. In this connection, the preventive use of
diuretics is ineffective [19,20]. To
date, the lack of necessary knowledge about the properties of commercial
albumin, its various variants, among doctors generates numerous discussions
with diametrically opposed views on the method of its application. Therefore, for effective and adequate
use of albumin clinicians need to have the following data: ·
The
degree of hydrophobicity of toxemia. ·
Indicator
of the reserve of albumin binding capacity. ·
Calculated
index of intoxication. Only
on the basis of the above indicators can be estimated: ·
Transport
function of albumin. ·
Detoxification
function of the liver. ·
Degree
of blocking of albumin binding centres. ·
In
combination with hydrophilic indicators of endotoxemia in dynamics, to estimate
the degree of endotoxicosis, the effectiveness of hemocorrection and
detoxification methods. For
effective use of albumin as an endogenous source of amino acids, the following
conditions are necessary: ·
No
circulating blood volume (CBV) deficit. ·
The
absence of diseases that disorder the process of absorption and enzymatic
activity of the stomach. ·
Preservation
of function protein synthesis in the liver. When
albumin is not used, or its use is not justified: 1.
In conditions of increased capillary permeability albumin very quickly leaves
the vascular bed and captures water, causing interstitial edema into the
different tissues. 2.
It is not recommended to use solutions of albumin in chronic nephrosis, because
albumin is quickly excreted by the kidneys. 3.
The use transfusion of album as a source of protein in patients suffering from intestinal
malabsorption,
chronic pancreatitis, chronic liver cirrhosis, body weight deficit after fasting,
serious CBV deficiency is not justified. 4.
It should also be known that the banal transfusion of protein preparations in
the multiple organ dysfunction syndrome does not correct hypoproteinemia due to
violations in the physiological chain of protein synthesis. Therefore, the
doctors argument: "for the correction of hypoproteinemia recommended
transfusion of albumin" sounds at least - incorrect!!!. In
what cases transfusion of albumin is adequate and safe: Albumin
should be used mainly in chronic hepatitis that accompanied by hypoalbuminemia,
edematous syndrome caused by hypoalbuminemia in the absence of severe
intoxication. These
practical recommendations for the effective use of albumin are based on
fundamental knowledge of the pathophysiology of blood
circulation, the studied features of metabolic disorders in tissues and should
be taken into account by the doctor. Conclusion To
date, there is no standardized and unified technology for the production of
commercial albumin. Produced by various pharmaceutical companies albumin has
different physical and chemical properties. A study of application of
commercial albumin should be conducted either within the same manufacturer or
by comparing the specific clinical effect of albumin which was produced by
different manufacturers. Unfortunately this approach of analysis is completely
absent in the medical literature. Therefore, the experience of using albumin by
some clinicians a priori is not a guide for others, and the discussions held on
the pages of medical journals, debates at conferences, thoughtless copying of
clinical of protocols without proper fundamental knowledge, there is scientific
ignorance and clinical illiteracy. References 1.
F
Bunn, C Lefebvre, A Li Wan Po, L Li, I Roberts, G Schierhout. The Albumin
Reviewers. Human albumin solution for resuscitation and volume expansion in
critically ill patients (2000) Cochrane Database Syst Rev 2: CD001208. http://dx.doi.org/10.1002/14651858.CD001208 2.
S
Finfer, R Bellomo, N Boyce, J French, J Myburgh, et al. A comparison of albumin
and saline for fluid resuscitation in the intensive care unit (2004) N Engl J
Med, 350: 2247-2256. http://dx.doi.org/10.1056/NEJMoa040232 3.
Haynes
G, Navickis R, Wilkes M. Albumin administration - what is the evidence of
clinical benefit? A systematic review of randomized controlled trials (2003)
European Journal of Anesthesiology 20: 771-793. https://doi.org/10.1017/S0265021503001273 4.
Boldt
J, Schöllhorn T, Mayer J, Piper S, Suttner S. The value of an albumin-based
intravascular volume replacement strategy in elderly patients undergoing major
abdominal surgery (2006) Anesth Analg 103: 191-199. 5.
Nicholson
JP, Wolmarans MR, Park GR. The role of albumin in critical illness (2000) Br J
Anaesth 85: 599-610. https://doi.org/10.1093/bja/85.4.599 6.
Wilkes
MM, Navickis RJ. Patient survival after human albumin administration—a
meta-analysis of randomized controlled trials (2001) Ann Intern Med 135: 149-164.
https://doi.org/10.7326/0003-4819-135-3-200108070-00007 7.
Van
der Heijden M, Verheij J, van Nieuw Amerongen GP, Groeneveld AB. Crystalloid or
colloid fluid loading and pulmonary permeability, edema, and injury in septic
and nonseptic critically ill patients with hypovolemia (2009) Crit Care Med 37:
1275-81). https://insights.ovid.com/crossref?an=00003246-200904000-00015 8.
Vincent
JL, Sakr Y, Reinhart K. Sepsis Occurrence in Acutely Ill Patients Investigators
is albumin administration in the acutely ill associated with increased mortality?
Results of the SOAP study (2005) Crit Care 9: 745-754. https://doi.org/10.1186/cc3895 9.
Schortgen
F, Girou E, Deye N, Brochard L. For the CRYCO Study Group the risk associated
with hyperoncotic colloids in patients with shock (2008) Intensive Care Med 34:
2157-2168. https://doi.org/10.1007/s00134-008-1225-2 10.
Dellinger
RP, Levy MM, Rhodes A, Annane D, Gerlach H, et al. International Surviving
Sepsis Campaign International guidelines for management of severe sepsis and
septic shock (2008) Crit Care Med 36: 296-327. https://doi.org/10.1097/01.CCM.0000298158.12101.41 11.
Myburgh
J, Cooper J, Finfer S, Bellomo R, Norton R, et al. Saline or albumin for fluid
resuscitation in patients with traumatic brain injury (2007) N Engl J Med 357:
874-884. https://doi.org/10.1056/NEJMoa067514 12.
Technology
Assessment: Albumin, Non-protein Colloid, and Crystalloid Solutions, 2000. Oak
Book, IL, USA. 13.
Tanzi
M, Gardner M, Megellas M, Lucio S, Restino M. Evaluation of the appropriate use
of albumin in adult and pediatric patients (2003) Am J Health Syst Pharm 60: 1330-1335.
https://doi.org/10.1093/ajhp/60.13.1330 14.
Dongping
Z, Abderrazzak D, and Ahmed H. Zewail. Femtosecond studies of protein-ligand
hydrophobic binding and dynamics: Human serum albumin (2000) Proc Natl Acad
Sci, USA 97: 14056-14061. https://doi.org/10.1073/pnas.250491297 15.
Rosenoer
VM, Oratz M, Rothschild MA. Albumin Structure, Function, and Uses (1977)
Oxford: Pergamon, USA. 16.
Yamasaki
K, Miyoshi T, Maruyama T, Takadate A, Otagiri M. Characterization of Region Ic
in Site I on Human Serum Albumin. Microenvironmental Analysis Using
Fluorescence Spectroscopy (1994) Biol Pharm Bull 17:1656-1662. https://doi.org/10.1248/bpb.17.1656 17.
Margaret
D, Shashank J, Nicholas H, Neil K and Alluru SR. Albumin and Furosemide
Combination for Management of Edema in Nephrotic Syndrome: A Review of Clinical
Studies (2015) Cells. 4: 622-630. https://doi.org/10.3390/cells4040622 18.
Davison
AM, Lambie AT, Verth AH, Cash JD. Salt-poor human albumin in management of
nephrotic syndrome (1974) Br Med J 1: 481-484. https://doi.org/10.1136/bmj.1.5906.481 19.
Dorhout
EJ, Roos JC, Boer P, Yoe OH, Simatupang T.A. Observations on edema formation in
the nephrotic syndrome in adults with minimal lesions (1979) Am J Med 67: 378-384.
https://doi.org/10.1016/0002-9343(79)90782-4 20.
Geers
AB, Koomans HA, Roos JC, Boer P, Dorhout Mees EJ. Functional relationships in
the nephrotic syndrome (1984) Kidney Int 26: 324-330. https://doi.org/10.1038/ki.1984.176 Albumin, Physical and
chemical properties,Indications,
ContraindicationsFeatures of Clinical Use of Albumin. Problems and Ways of Decision Solutions
Andrey Belousov
Abstract
Full-Text
Keywords