Research Article :
Rubel MD,
Sheik Akij MD and Shahidul Islam MD This research work designates of interaction and complexation studies of Cefixime with essential metal. Cefixime involved the third generation drug of penicillin. In vitro analysis, Cefixime must be interacted with metal like Mg2+and Mn2+. At pH 7.4, this study was done in different ratios of cefixime with metal and antacid both at room temperature 25°C. By this study, it is investigated that drug Cefixime is complexed with metal as well as antacid which is confirmed by jobs plot. This experiment was carried out by using ultra violet spectrophotometer. The microbial sensitivity test is important to know whether there is any change in the effectivity of Cefixime after the interaction with metals. There was a remarkable change in the effectivity of Cefixime and its complexes. This research work confirms that there was interaction between Cefixime with Metals like Mg2+and Mn2+which was confirmed by Jobs plot method by spectrophotometric assay. Drug-drug,
drug-metal interaction
is a common phenomenon. A drug interaction is said to occur when the effects of
one drug are changed by the presence of another drug, herbal medicine, food,
drink or by some environmental chemical agent. These unwanted and unsought-for
interactions are adverse and undesirable but there are other interactions that
can be beneficial and valuable, such as the deliberate co-prescription of
antihypertensive drugs and diuretics in order to achieve antihypertensive
effects possibly not obtainable with either drug alone. The mechanisms of both
types of interaction, whether adverse or beneficial, are often very similar [1]. There
are different types of drug interactions: Drug-drug interact ions, Drug-herbal interact ions,
Food-drug interact ions etc. Drug interactions are complex and chiefly
unpredictable. A known interaction may not occur in every individual. This can
be explained because there are several factors that effects the likelihood that
a known interaction will occur. Zinc
is an important co-factor for several enzymatic reactions in the human body,
vitamin B12 has cobalt atom and its core, and hemoglobin contains iron. Like
Cu, Mn, Se, Cr, Mo are all trace elements, which
are important in the human diet. Another subset of metals include those used in
therapeutically in medicine, Al, Bi, Au, Ga, Li and Ag are all part of medical armamentarium
[2-4]. Humans
need a certain amount of certain metals to function normally. Most metals are
used as cofactors or prosthetics in enzymes, catalyzing specific
reactions and serving essential roles. Anemia symptoms are caused by lack of a
certain essential metal. Anemia can be associated with malnourishment or faulty
metabolic processes, usually caused by a genetic defect. The metal complexes
can be utilized for the transport of selected organic chemotherapeutic drugs to
target organs, or for the decorporation of those toxic organic compounds which
are able, before or after metabolic activation of reacting with metals or 1:1
complexes. It
is emphasized that degree to which metal ions interact in vivo should employ
the conditional constants which take into account competition from other ions
specially Ca2+, H+ and OH-. The genotoxic
consequences of the virus chemical factors involved in chelation, along with
examples; kinetics, stabilization or oxidation state, lipophilicity, the
mixed ligand formation, are discussed. Cefixime is a semi-synthetic, moderate
spectrum of penicillin group of antibiotic. Cefixime is an
antibiotic that is used for the treatment of a variety of bacterial infections,
skin and urinary tract infections,
pneumonia,
and strep throat. It binds to one of the penicillin binding proteins which
inhibit the final transpeptidation step of the peptidoglycan synthesis in the
bacterial cell wall, thus inhibiting biosynthesis and arresting cell wall
assembly resulting in bacterial cell death. Cefixime is a broad spectrum
antibiotic and is commonly used to treat bacterial infections of the ear,
urinary tract, and upper respiratory tract [5-10]. In
this study, all the chemical substances, reagents used here were pure and were
kept under sorted under suitable conditions. Preparation of stock solution:In this study,
250 ml of 1×10ˉ2 stock solution of
Cefixime was measured by 0.16902 gm of Cefixime in 250 ml of demineralised water
in a 250 ml volumetric flask. This solution was further diluted to expected
concentration by using buffer solution. Preparation
of standard curve of Cefixime: In this study, Cefixime
stock solution at pH 7.4 and concentration 1×10-5M was
mixed in different concentrations like: 9×10-5M, 8×10-5M,
7 ×10-5M, 6×10-5M, 5×10-5M, 4×10-5M,
3×10-5M, 2×10-5M, 1×10-5M. The absorbance volume
of the solutions was determined at 256 nm by UV spectrometer [13]. Table
1: List of chemicals and reagents. All
the equipment and instruments used throughout the study are given in the following
table along with their source. Table
2: List of instruments and equipment. Table
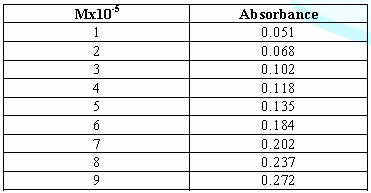
3: Standards curve of Cefixime. From
the above Table, we can observe that the absorbance of Cefixime increases with
increasing concentration according to Beer Lamberts law. Table
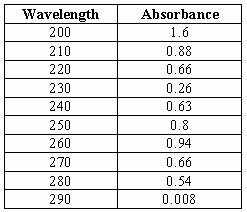
4: Absorbance of Cefixime at different
wavelength. From the above table we
can observe that the absorbance of Cefixime varies at different wavelength. Table
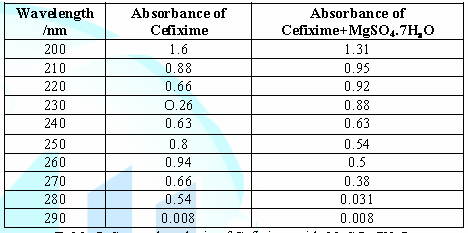
5: Spectral analysis of Cefixime with MgSO4.7H2O. From
the above table we can observe that the absorbance of Cefixime is different
when it interacts with MgSO4.7H2O due to drug metal
interaction. Table
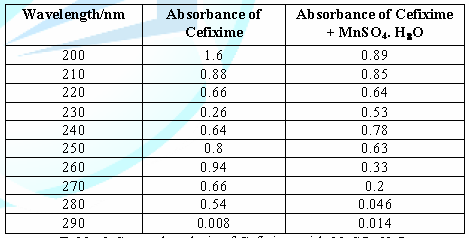
6: Spectral analysis of Cefixime with MnSO4.H2O. From
the above table, we can observe that the absorbance of Cefixime
is different when it interacts with MnSO4.H2O. This
table shows that absorbance of cefixime is quite different from absorbance of
Cefixime and metal complexes. The intensity of the peak of Cefixime changes
remarkably i.e. absorption characteristics are altered due to interaction but
the position of the compound does not shift. Interaction between drug and metal
may lead to form complexes which have different light absorption capacity and
spectrum pattern is altered. Effect of metals on Cefixime by Jobs method of
continuous variation: The
molar ratios of the complexes of Cefixime with metal salts were estimated by
jobs method of continuous variation. The observed absorbance values were
measured in pH 7.4 at various concentrations (1×10-5 to 9
× 10-5M) of Cefixime and metal salts at 2310 nm. The Jobs plots at
pH 7.4 were obtained by plotting absorbance difference against the mole
fraction of the drug which is presented in the following table. Table
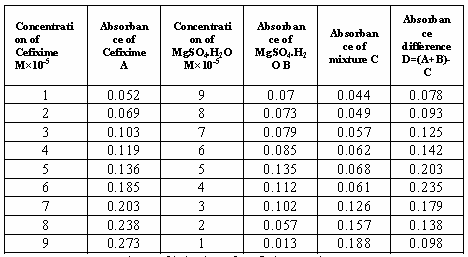
7: Values of job plot of Cefixime and MgSO4.7H2O. From
the above table we can observe that Cefixime forms strong 1:1 complexes with manganese Sulfate mono
hydrate which is indicated as ^ shaped curve which indicates confirm
interaction and complexation with drug and metal. Table
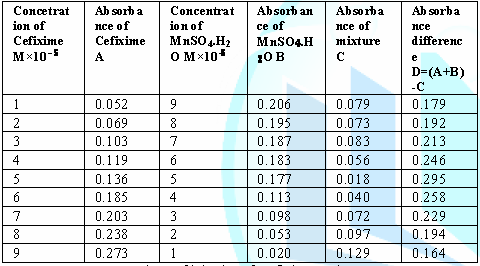
8: Values of job plot of Cefixime and MnSO4.H2O. From
the above table we can observe that Cefixime forms strong 1:1 complexes with
manganese Sulfate mono hydrate which is indicated as ^ shaped curve which
indicates confirm interaction and complexation with drug and metal. Table
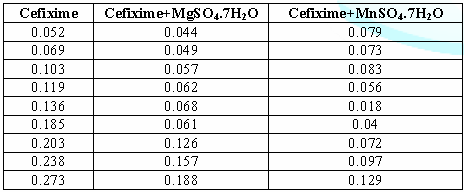
9: Combined absorbance of drug with different
metal. The
above table shows that the absorbance of Cefixime differs from the absorbance
of Cefixime + MgSO4.7H2O and Cefixime + MnSO4.H2O
due to interaction and complexation
with antibiotic and metals. In
this research work, the interaction of an important antimicrobial drug,
Cefixime and MgSO4.7H2O and also Cefixime, MnSO4.H2O
at 7.4 by a variety of physical method like inspection of spectral behavior,
Jobs method of continuous variation. From spectral study, it has been observed
that Cefixime gives a sharp peak at 256 nm. When MgSO4.7H2O
and MnSO4.H2O salt mixed with Cefixime at 1:1 ratio, the
intensity of the peak changes remarkably specifically absorbance decreases
sharply. Thats why absorption characteristics are altered due to interaction
but the position of the compound does not shift at all. The Jobs plot has given
the molar ratio of complexes of Cefixime and MgSO4.7H2O
and also Cefixime, MnSO4.H2O. At pH 7.4
Cefixime forms strong 1:1 complexes with metals MgSO4.7H2O
and MnSO4.H2O indicated as ^ shaped curves. These curves
indicate strong kinetics of complexation between Cefixime with MgSO4.7H2O
and Cefixime with MnSO4.H2O. When drug individually act
with metals MgSO4.7H2O and MnSO4.H2O
curve of their absorbance are verify. By this research work, it helps in the
study of selection the best dosage form for better treatment which can be
justified by the future in vivo analysis. And
definitely very important in adjusting the effective dose and dose ranges. 1.
Meletiadis
J, Mouton WJ, Meis FJ and Verweij EP. In vitro drug interaction modeling of
combinations of azoles with terbinafine against clinical Scedosporium
prolificans isolates (2003) Antimicrobial Agents Chemotherapy 1: 106-117. https://doi.org/10.1128/aac.47.1.106-117.2003 2.
Chou
CT. Theoretical basis, experimental design, and computerized simulation of
synergism and antagonism in drug combination studies (2006) Pharmacological Reviews
58: 621-681. https://doi.org/10.1124/pr.58.3.10 3.
Herfindal
ET and Hirschman JL. Clinical pharmacy and therapeutics (1979) Williams &
Wilkins, United States. 4.
Zhang
L, Reynolds KS, Zhao P and Huang SM. Drug interactions evaluation: An
integrated part of risk assessment of therapeutics (2010) Toxicology Applied Pharmacology
243: 134-145. https://doi.org/10.1016/j.taap.2009.12.016 5.
Butt
EM, Nusbaum RE, Gilmoult TC and Didio SL. Trace metal patterns in disease
states: I. Hemochromatosis and refractory anemia (1956) American J Clinical Pathology
26: 225-242. https://doi.org/10.1093/ajcp/26.3.225 6.
Martell
AE and Smith RM. Critical stability constants (1974) Plenum Press, New York. 7.
Davis
JA and Leckie JO. Surface ionization and complexation at the oxide/water
interface II. Surface properties of amorphous iron oxyhydroxide and adsorption
of metal ions (1978) J Colloid Interface Science 67: 90-107. https://doi.org/10.1016/0021-9797(78)90217-5 8.
Okamoto
OK, Pinto E, Latorre LR, Bechara EJ and Colepicolo P. Antioxidant modulation in
response to metal-induced oxidative stress in algal chloroplasts (2001)
Archives of Environmental Contamination and Toxicology 40: 18-24. https://doi.org/10.1007/s002440010144 9.
Tsuji
A, Terasaki T, Tamai I and Hirooka H. H+ gradient-dependent and
carrier-mediated transport of cefixime, a new cephalosporin antibiotic, across
brush-border membrane vesicles from rat small intestine (1987) J Pharmacology
Experimental Therapeutics 241: 594-601.
https://doi.org/10.1016/0006-2952(87)90369-8 10.
Furman
WL, Crews KR, Billups C, Wu J, Gajjar AJ, et al. Cefixime allows greater dose
escalation of oral irinotecan: a phase I study in pediatric patients (2006) J Clinical
Oncology 24: 563-570. https://doi.org/10.1200/jco.2005.03.2847 11.
Frankenberger
W and Dick WA. Relationships between enzyme activities and microbial growth and
activity indices in soil (1983) Soil Science Society of America Journal 47: 945-51. 12.
Islam
MDS and Sabekun MB. An interaction study of metal ions complexation with
cephradine on its antibacterial activity (2018) IRJPMS 1: 17-20. 13.
Islam
MDS and Nobina A. In-Vitro interaction of cefpodoximeproxetil with different
essential metal, antacids and investigation of antimicrobial activity (2019)
IRJPMS 2: 70-73. *Corresponding author: Islam SM, Assistant Professor,
Department of Pharmacy, University of Science and Technology Chittagong (USTC)
Bangladesh, Email: s_i_liton@yahoo.com
Citation: Rubel MD, Akij MDS and Islam MDS. Complexation and interaction study of
antibiotic cifixime with essential metals by spectrophotometric method (2019) Edelweiss
Pharma Analy Acta 1: 12-15. Cefixime,
Complexation, Interaction, Jobs PlotComplexation and Interaction Study of Antibiotic Cifixime with Essential Metals by Spectrophotometric Method
Abstract
Full-Text
Introduction
Materials
and Methods
Preparation of metal solution: In
this study, 0.1M metal solution, Magnesium sulphate hepata hydrate, MgSO4.7H2O
(0.24648gm) was measured perfectly as well as the last solutions were 0.01 M
concentration.
Preparation of buffer solution: In
this study, for the preparation of buffer solution, firstly 8.06 gm of Na2HPO4
was mixed in demineralized water with 1.05 gm of NaH2PO4
and then pH 7.4 was confirmed [12].
Results
and Discussion
Conclusion
References
Keywords