Review Article :
There is a continued interest to screen plant
extracts for their antioxidant properties, in light of the fact that
antioxidant activities parallel anticancer activities, amongst their ability to
combat other diseases. Cancer is one of the diseases that has a high mortality
status in developed countries and is on the rise in developing countries. Plant
extracts have been tested for their antimicrobial, anticancer, antidiabetic,
insect repellant and a range of other biological activities. Since 1990s,
antioxidant research has expanded significantly, due to its potential benefits
in disease prevention and health promotion. Guyana, a country located on the
mainland of South America and whose rich diverse flora needs continual
screening for plants with a range of pharmaceutical and medicinal activities of
which, antioxidant is one. In addition, the isolation of known and unknown
natural antioxidants may contribute to novel drug discovery. This article is a
mini review of plants/plant extracts that have exhibited antioxidant
properties. Antioxidants are chemical
compounds or mixture of compounds, which when present in low concentrations are
used to prevent the oxidation of lipids, sugars and proteins and DNA that can
generate aldehydes, ketones, esters and other products that can be harmful to
living systems. Antioxidants can be synthetic or natural. Synthetic
antioxidants include Butylated Hydroxyl Anisole (BHA), Butylated Hydroxyl Toluene (BHT), Tert-Butyl
Hydroquinone (TBHQ) and propyl gallate (PG) etc. [1].
Natural antioxidants are those that can be obtained from fruits, vegetables and
plant extracts. There is an increasing interests to use plant extracts as
antioxidant agents. Table 1 shows
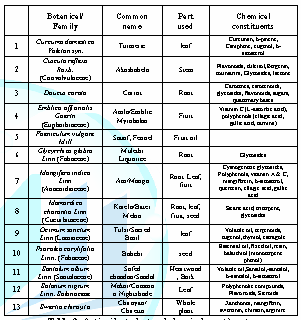
some plants that have rich antioxidant profile, whereas Table 2 shows the chemical constituents of some antioxidant plants.
Natural antioxidants may function (a) as reducing agents, (b) as free radical
scavengers, (c) as complexers of pro-oxidant metals, and (d) as quenchers of
the formation of singlet oxygen. However, the major value is in their primary
antioxidant activity as free radical acceptors and as chain breakers. Free radicals
are usually produced in normal or pathological cell metabolism. Reactive Oxygen
Species (ROS) react with free radicals to become free radicals themselves. ROS
include free radicals such as superoxide anion radicals, hydroxyl radicals,
non-free radical species and singlet oxygen [2-5]. Excess generation of ROS,
induced by various stimuli and which exceed the antioxidant capacity of the
organism can lead to various pathophysiological processes such as diabetes,
cancer, inflammation, genotoxicity, Alzheimers disease and cataracts,
retinopathy, rheumatism, skin disease porphyria and senile dementia stroke [6-8]. Antioxidants usually react with reactive free
radicals to destroy them by accepting or donating electron(s) to eliminate the
radical or they may indirectly decrease the formation of free radicals.
Antioxidants also act by forming complexes with metals. Human cells protect
themselves against enzymatic and non-enzymatic antioxidant systems against free
radical damage. However, these protective antioxidant mechanisms may not be
enough to prevent severe or continued antioxidant stress [9]. Hence, natural or
synthetic antioxidants are necessary. In nature, there is a wide variety of natural
antioxidants which are different in their chemical composition, physical and
chemical properties. These include enzymes such as Superoxide dismutase,
catalase etc., high molecular weight compounds such as protein like albumin,
transferrin, ceruplasmin, low molecular weight compounds such as tocopherol,
quinines, bilirubin, ascorbic acid, uric acid etc, minerals such as selenium,
copper, manganese, zinc etc. vitamins such as vitamin A,C and E and plant
antioxidants. Also, the flavonoids (flavanols, isoflavones, flavones, catchins,
flavanones), cinnamic acid derivatives, coumarins, tocopherols, and
polyfunctional organic acids. Some of these are shown in Figure 1 Screening of plants for antioxidant activities can be
established via various in vitro methods
such as DPPH, Nitric oxide method, DMPD, ABTS, ORAC, TBARS assays1-8, [20-25]
etc. Guyana
has a richly biodiversified flora and medicinal studies such as antimicrobial [10-18],
Antidiabetic [19] have received increasing attention. However, there are few
unpublished work on antioxidant and anticancer activities. It’s highly
imperative that research proliferate with regards to anticancer and antioxidant
activities as there are an alarming increase in the deadly cancer disease
worldwide and in Guyana. Plant parts such as stems, leaves and fruits rich in
antioxidant properties are good in combat against cancer. Also, the chromatographic purification of the crude
plant extracts from the Guyanese flora can lead to known and unknown natural
products, whose antioxidants properties can be investigated and compared with
the crude plant extracts. This forms the basis for novel antioxidant drugs
discovery. Few researches in Guyana have done on the isolation of natural
products from plants of the Guyanese flora. The isolation of natural products
from Montricardia arborescens and Passiflora edulis, two plants from the Guyanese flora, suspected to have
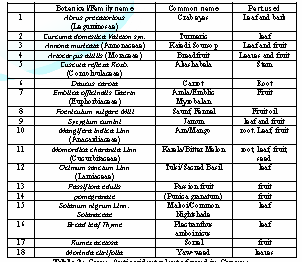
antioxidant properties have been pursued. Table
3 shows some plants with suspected antioxidant properties, based on
folklore from the flora of Guyana. There chemical constituents needs
investigation. Table 1: Some medicinal plants with antioxidants activity. The
antioxidant activities of plant extracts, fruits and vegetables are well
documented [1-9], [20-25]. Thirty (30) plants aqueous extracts were
investigated for their antioxidant properties via several methods such as DPPH,
ABTS radical scavenging capacity assay, oxygen radical absorbance capacity
(ORAC) assay, superoxide dismutase (SOD) assay, and ferric reducing antioxidant
potential (FRAP) [20]. In addition, the Total phenolic content was determined
by the Folin−Ciocalteu
method,
(FCM). Results showed that oak (Quercus
robur), pine (Pinus maritima),
and cinnamon (Cinnamomum zeylanicum)
aqueous extracts possessed the highest antioxidant activities and thus could be
potential rich sources of natural antioxidants. A significant relationship
existed between antioxidant capacity and total phenolic content, indicating
that phenolic compounds are the major contributors to the antioxidant
properties of these plants [20]. The
antioxidant activities of the methanol extracts from the leaves and stems of Celtis
africana
(Ulmaceae)
were assessed in an effort to validate the medicinal potential of the
subterranean part of the herb. The antioxidant activity and phenolic contents
of the stem as determined by the DPPH, proanthocyanidins, total phenols, the
flavonoids, and total flavonols were higher than that of the leaves [21]. Table 2: Antioxidant plant and chemical constituents. Table 3: Some Antioxidant plants found in Guyana. Three
plant foods, namely, drumstick leaves (Moringa
oleifera), mint leaves (Mentha
spicata) and carrot tuber (Daucus
carota) ethanolic extracts extracts were analyzed for their antioxidant
activity. The antioxidant activity of extracts was evaluated according to the
amount of malonaldehyde (MDA) formed by the FeSO4-induced oxidation
of linoleic acid and a high PUFA oil (sunflower
oil) at 37 °C in Trizma-buffer (pH 7.4). The antioxidant activity of the
extracts from mint leaves and carrot was higher at pH 9 than pH 4, while that
of drumstick extract remained the same under both pH conditions [22]. The
antioxidative activity of 92 phenolic extracts from edible and nonedible plant
(berries, fruits, vegetables, herbs, cereals, tree materials, plant sprouts,
and seeds) was examined by autoxidation of methyl linoleate method [23]. The
total phenolics content in the extracts was determined spectroscopically via
the Folin−Ciocalteu assay. For the edible plants, high antioxidant activity and
high total phenolic content, expressed as gallic acid equivalents, were found
in berries (GAE > 20mg/g), such as aronia and crowberry. Apple extracts (two
varieties) also showed strong antioxidant activity, despite low total phenolic
contents (GAE < 12.1 mg/g). For nonedible plant species, high antioxidant
activities were found in willow bark, spruce needles, pine bark, cork, and
birch phloem, and in some medicinal plants such as heather, bog-rosemary,
willow herb, and meadowsweet. Potato peel and beetroot peel extracts also
showed strong antioxidant effects [23]. Natural
antioxidants from plants can protect against DNA oxidative damage human
lymphocytes induced by hydrogen peroxide, H2O2. Thus, six
herbaceous plants, including Bidens alba (BA), Lycium chinense (LC), Mentha
arvensis (MA), Plantago asiatica
(PA),Houttuynia cordata (HC), and Centella asiatica (CA) were investigated
for their antioxidant activities. The plants were found to be rich in flavonols, such as
myricetinin BA, morin in MA, quercetin in HC, and kaemperol in CA. In addition,
polyphenol were abundant in BA and CA. Antioxidant efficacy was determined by
the inhibition percentage of conjugated diene formation in a linoleic acid
emulsion system and by trolox-equivalent antioxidant capacity (TEAC) assay. The
acidic methanolic extract of PA, induced the best conjugated diene formation
inhibition percentage. For TEAC, the best antioxidant activity was generated
from the acidic methanolic extract of HC [24]. Extracts
from Brazilian plants, belonging to 16 species of 5 different families (71
extracts) were tested for their antioxidant activities. Ginkgo
biloba
and rutin, commonly used as antioxidants for medical purposes, were used as
standards. The ethanol extracts of
plants belonging to the Verbenaceae family showed lower EC50 values
than the other plant extracts. It was found that the more polar partitions
(ethyl acetate and n‐butanol) are those that generally have higher antioxidant
activity [25]. Plant
extracts, fruits and vegetables have indeed shown to possess antioxidant
activities. Research needs to be continued for the search of plant with
interesting antioxidant effects. In addition, the isolation of known and
unknown natural antioxidants will form the platform for novel drug discovery.
In this regards, diverse rainforest tropical flora in a diverse ecosystems from
Guyana, needs further herbal exploration and commercialization, in addition to
their impetus for eco-tourism. Only a couple of reports on the isolation of
natural products from two plants suspected to have antioxidant properties have
been reported here. Apart from antioxidant drugs, new drugs such
as ant-AIDS, anti-cancer, anti-diabetes, anti-arthritis and anti-alzheimers still awaiting
discovery. 1. Jayathilakan
K, Sharma GK, Radhakrishna K and Bawa AS. Antioxidant potential of synthetic
and natural antioxidants and its effect on warmed-over-flavour in different
species of meat (2007) Food Chem 105: 908-916. https://doi.org/10.1016/j.foodchem.2007.04.068 2. Halliwell
B. How to characterize an antioxidant: an update (1905) Bioch Soc Sym 61:
85-91. https://doi.org/10.1042/bss0610073 3. Squadriato
GL and Peyor WA. Oxidative Chemistry of nitric oxide: the role of superoxide,
peroxynitrite, and carbon dioxide (1998) Free radicals Biol Med 25: 392-403. https://doi.org/10.1016/s0891-5849(98)00095-1 4. Yildrim
A, Oktay M and Bilaloglu V.The antioxidant activity of the leaves of Cydonia
vulgaris (2001) Turkish J Med Sc 31: 23-27. 5. Gulcin
I, Oktay M, Kufrevioglu IO and Aslan A. Determination of antioxidant activity
of lichen Cetraria islandica (L) (2002)
Ach J Ethnopharmacol 79: 325-329. https://doi.org/10.1016/s0378-8741(01)00396-8 6. Kourounakis
AP, Galanakis D, and Tsiakitzis K. Synthesis and pharmacological evaluation of
novel derivatives of anti-inflammatory drugs with increased antioxidant and
anti-inflammatory activities (1999) Drug Dev Res 47: 9-16. https://doi.org/10.1002/(sici)1098-2299(199905)47:1<9::aid-ddr2>3.0.co;2-9 7. Gulcin
I, Buyukokuroglu ME, Oktay M and Kufrevioglu IO. On the in vitro antioxidant
properties of melatonin (2002) J Pineal Res 33: 167-171. https://doi.org/10.1034/j.1600-079x.2002.20920.x 8. Gulcin
I, Buyukokuroglu ME, M. Oktay and I.O. Kufrevioglu. Antioxidant and analgesic
activities of turpentine of Pinus nigra
Arn. Subsp. Pallsiana (Lamb) (2003)
Holmboe J Ethnopharmacol 86: 51-58. https://doi.org/10.1016/s0378-8741(03)00036-9 9. Lu
JM, Lin PH, Yao Q and Chen C. Chemical and molecular mechanisms of
Antioxidants: Experimental approaches and model systems (2010) J Cell Mol Med
(Berl) 14: 840-860. https://doi.org/10.1111/j.1582-4934.2009.00897.x 10. Jagessar
RC and Mohamed N, Antimicrobial activity of selected plants extracts from
Guyanas flora (2010) J Pure and Appl Microbio 4: 533-540. 11. Jagessar
RC and Allen R. Antimicrobial Potency of the Aqueous Extract of leaves of Terminalia catappa (2011) Aca Res Int 362-371. https://doi.org/10.20959/wjpps20179-10010 12. Jagessar
RC, Mars A, and Gomathigayam S, Selective Antimicrobial properties of Leaf
extract of Samanea Saman against Candida
albicans, Staphylococcus aureus and
Escherichia coli using several microbial techniques(2011) J American Sci 7: 108-119. http://dx.doi.org/10.13040/IJPSR.0975-8232.4(6).2114-20 13. Jagessar
RC, Mars A, Gomes G, Leaf extract of Smilax
schomburgkiana exhibit selective antimicrobial properties against
pathogenic microorganisms (2009) Life Sci J 6: 76-83. 14. Jagessar
RC, Mohammed A and Gomes G, An evaluation of the antibacterial and antifungal
activity of leaf extracts of Momordica
Charantia against Candida albicans,
Staphylococcus aureus and Eschericia
Coli (2008) J Nature and Sci 6: 1-14. 15. Jagessar
RC, Rodriques A, Prasad K, Husain A, Kanhai V,et al,. An investigation of the
hypoglycemic effect of the aqueous extract of the fruits of Psidum Guajava, Averrhoa Bilimbi and the
peel of Tamarindus indica in
Normoglycemic guinea pigs( 2018) WJPPS 7: 77-101. https://doi.org/10.4172/2325-9604-C3-032 16. Jagessar
RC, Ramchartar N and Spencer O. Fruits and Edible Plants, in Fruit and Pomace
Extracts: Biological Activity, Potential Applications and Beneficial Health
Effects (2015) Jason P. Owen (Ed) Nova Science Publisher, USA. 17. Jagessar
RC, Hafeez, Chichester, Crepaul Y. Antimicrobial Activity of the Ethanolic and
Aqueous Extract of Passion Fruit (Passiflora
edulis Sims), in the absence and presence of Zn (OAc)2.2H2O
(2017) World J Pharm Pharmaceuti Sci 6: 230-246. https://doi.org/10.20959/wjpps20179-10010 18. Jagessar
RC and Hope S. Antimicrobial Activity of the Uncombined and Combined Aqueous
Extract of Phyllanthus Acidus,
Sphagneticola Trilobata Leaves and Doliocarpus
Dentatus Bark against Selective Pathogenic Microorganisms in the absence
and presence of Zn2+ cations (2016) World J Pharm Pharmaceuti Sci 5:
58-71. https://doi.org/10.4172/2329-6631-C2-029 19. Jagessar
RC, Rodrigues A, Prashad K, Husain A, Kanhai V,et al,. (2018) An investigation
of the hypoglycemic effect of the aqueous extract of the fruits of Psidium Guajava, Averrhoa bilimbi and
the peel of Tamarindus indica in normoglycemic
guinea pigs (2018) World J Pharm Pharmaceuti Sci 7: 77-101. 10.4172/2325-9604-C3-032 20. Dudonné
S , Vitrac X, Coutière P, Woillez M and Mérillon JM. Comparative Study of
Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial
Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays (2009) J. Agric. Food
Chem 57: 1768-1774. https://doi.org/10.1021/jf803011r 21. Adedapo
AA, Jimoh FO, Afolayan AJ and Masika PJ. Antioxidant Properties of the Methanol
Extracts of the Leaves and Stems of Celtis Africana (2009) Rec Nat Prod 3:
23-31. 22. Arabshahi
DS, Devi DV and Urooj A. Evaluation of antioxidant activity of some plant
extracts and their heat, pH and storage stability (2007) Food Chem 100:
1100-1105. https://doi.org/10.1016/j.foodchem.2005.11.014 23. Kähkönen
MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, et al,. Antioxidant Activity of
Plant Extracts Containing Phenolic Compounds (1999) 47: 3954–3962. https://doi.org/10.1021/jf990146l 24. Lin
KH, Yang YY, Yang CM, Huang MY, Lo HF, Liu KC, Lin HS, Chao PY. Antioxidant
activity of herbaceous plant extracts protect against hydrogenperoxide-induced DNA
damage in human lymphocytes (2013), BMC Res Notes 6: 490. https://doi.org/10.1186/1756-0500-6-490 25. Luciana L, Mensor
FS, Menezes GG, Leitão AS. Reis T C, et al,. Screening of Brazilian plant
extracts for antioxidant activity by the use of DPPH free radical method (2001)
15: 127-130. https://doi.org/10.1002/ptr.687 Corresponding author: Jagessar RC, Department of
Chemistry, Faculty of Natural Sciences, University of Guyana (USTC), Georgetown,
Guyana, Email: raymondjagessar@yahoo.com Citation: Jagessar RC. Antioxidant properties
of plant extracts (2019) Edelweiss Pharma Analy Acta 1: 15-18. Screen plant extracts, Antioxidant activities, Anticancer
activities, Antidiabetic activities.Antioxidant Properties of Plant Extracts
Abstract
Full-Text
Introduction
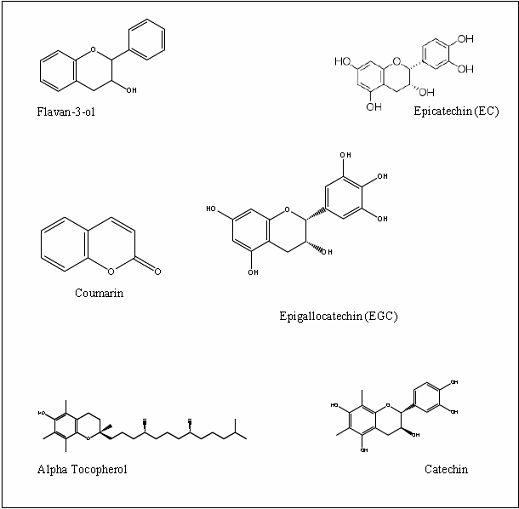
Figure 1: Some
antioxidants isolated from plants.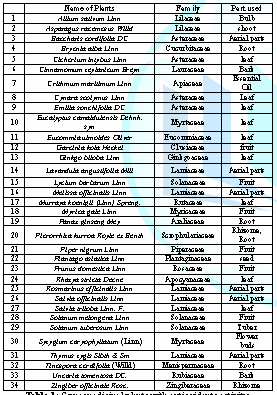

Conclusion
References
Keywords