Introduction
Corrosion is a noticeable phenomenon regarding the metal manufacturing and applications of such metals. The metallic corrosion is usually modified by the corrosive causing properties of some natural compounds [1]. Mineral oil is a mixture of hydrocarbons since it is composed with some vehement corrosive compounds such as elemental sulfur, active sulfur compounds, organic acids and salts [2-6]. Usually the corrosion is a term which is defined as the destruction of a material due to the chemical of electrochemical process as results of the interaction between such material and the surrounded environment also its found as a chemical oxidation process for the metallic corrosion since the metallic corrosion can be further categorized into several corrosion types such as general corrosion or rust, pitting corrosion, galvanic corrosion and stress corrosion including some specific features for each sub category of corrosion [1-5]. The surrounded environment plays a major role in the cause of any corrosion on such metal through creating a corrosive friendly background. The essential factor for the formation of the corrosion the relevant metal need to expose to both water and oxygen containing environment or another oxidizing agent which is stronger than Fe2+ while the improving the process by the acids and salts presence in the surrounding environment [1-9]. The impacts of such corrosive properties of mineral oils on the corrosion of some specific metal and nature of metallic corrosion due to the effects of several chemical compounds have been investigated and interpreted much important results from most of previous chemical and petroleum engineering researches regarding both whilom and recent studies.
In the current research there were aspired to speculate the performances of stainless steels against the mineral oils when comparing with other metals, the contribution of such corrosive properties of mineral oils together in the causing of the metallic corrosion at general environmental conditions, the visible appearances of the formed corrosion compounds on the metal surfaces due to the interaction between the mineral oils and such metals and the variations of the surfaces properties of metal due to the formation of the corrosion such as the hardness.
Materials and
Methodology
The selected ferrous metals and their applications in the mineral oil industry have been shortlisted in the Table 1.
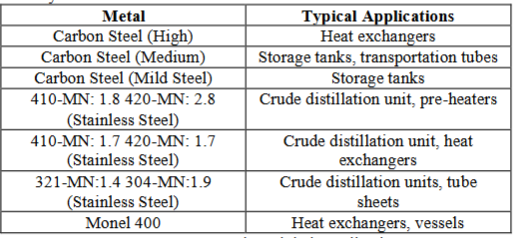
Table 1: Ferrous metals and their applications.
The chemical compositions of each type of metal was detected by the XRF detector and according to the working principles of XRF detector able to detect the all metallic elements composed in the crowing metal and some of nonmetals excluding carbon as a percentage.
According to the usage types of mineral oils there were selected two different types of mineral oils namely as Murban and Das Blend. Das Blend is known as a “sour” mineral oil or “sour” crude oil because of the relatively high sulfur content of Das Blend and Murban is a mediocre crude oil or mineral oil which is used in several refineries in the world. Those crude oils may slightly or extensively different in their chemical compositions. The elemental sulfur contents, mercaptans sulfur contents, acidity and salt contents of both crude oils were investigated because of the corrosive tendency of such compounds. The standard test methodologies for the testing of corrosive properties in both mineral oils have been summarized in the Table 2.
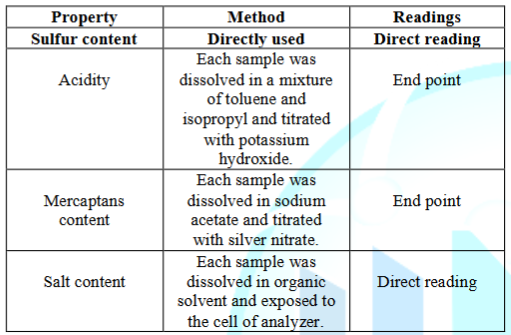
Table 2: Test methodologies for the corrosive properties of mineral oils.
A set of similar sized metal coupons was prepared from each type of metals as six metal coupons from each type of metal and the dimensions and weights of such metal coupons were measured according the requirements of mathematical calculations. Those metal coupons were immersed separately and homogeneously by considering both the mineral oil and the type of metal as shown in the Figure 1.

Figure 1: Metal coupons and apparatus setup.
After 15 days from the immersion one metal coupon was taken out from each mineral oil container as representing both metal type and mineral oil and the corrosion rates of such metal coupons were determined by the relative weight loss method [10]. The same procedure was repeated for another two sets of metal coupons after 30 and 45 days from the immersion. The corroded surfaces were observed by the 400X of an optical microscope and the corroded metal surfaces were cleaned by sand papers and isooctane to determine the weight losses of such metal coupons due to the corrosion. The mathematical expression and the terms of that expression have been given in the below.
CR=W*k/(D*A*t)…………….(1)
Where
W = weight loss in grams
k = constant (22,300)
D = metal density in g/cm3
A = area of metal piece (inch2)
t = time (days)
CR= Corrosion rate of metal piece
The microscopic analysis was performed as an identification stage of the formation of the corrosion and partial requirement of the weight loss methods to make free the metallic surface from corroded particles. The surface of each metal coupon was observed under the 400X lens of a laboratory optical microscope before immersion in the mineral oils, after taking out from the mineral oils and while determining the corrosion rate of such metal coupon as necessity.
Based on the observations of invisible weigh losses of some of metal coupons and as a confirmation stage of the formations of the metallic corrosion the decayed ferrous concentrations into crude oil samples which were exposed to the carbon steels and stainless steels also the decayed copper concentrations into crude oil samples which were interacted with Monel metal coupons were tested by the Atomic Absorption Spectroscopy (AAS). According to the methodology of the sample preparation 1 ml of each crude oil sample was diluted with 9 ml of 2-proponol and filtered.
Finally the deductions of the initial hardness of metal coupons due to the corrosion were tested by the Vickers hardness tester as a detection stage of the surface changes of metal coupons due to the corrosion. The initial hardness and the hardness after formation of the corrosion on the surface were tested in each metal coupon. According to the test methodologies of the Vickers hardness tester hardness of at least three points on the metal surface were tested at once and the average value was interpreted the hardness of such metal at that moment.
Results and Discussion
According to the results of the XRF detector the elemental compositions of the ferrous metals are given in the Table 3.
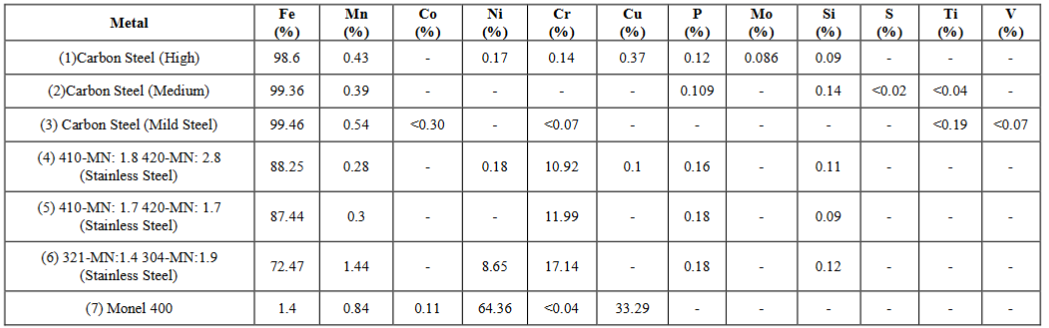
Table 3: Chemical compositions of ferrous metals.
According to the obtained results for the chemical compositions of the ferrous metals higher ferrous concentrations from carbon steels, moderate ferrous concentrations form stainless steels and a trace amount of ferrous were found. In addition some other metals were found in trace amounts especially in stainless steels such as nickel and copper. The major objectives of the doping of some trace metals and non-metal with some ferrous metals are the enhancements of the strength, hardness and the throttling of the corrosive tendency of such ferrous metals [1,3]. The prominent and efficacy phenomenon regarding the stainless steels is that the ability of the formation of self- corrosive protection layer on the metal itself against any kind of corrosive environment when having a chemical composition of at least 12% of chromium and sufficient amount of nickel [1,3,5].
The results for the analysis of the corrosive properties of both Murban and Das Blend mineral oils are summarized in the Table 4.
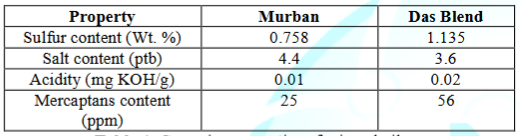
Table 4: Corrosive properties of mineral oils.
Above results showed relatively higher contents of both elemental sulfur and Mercaptans in Das Blend crude oils than the Murban crude oil approximately in twice as defined the Das Blend as a “sour” crude oil or more than that. Usually the due to the reaction between elemental sulfur and water presence in crude oils the metallic corrosion is happened and the process is known as in term “localized corrosion” and the reaction between the active sulfur compounds which are having fraction or some active functional group and metals is known as the process of “sulfidation” [2,6]. Most of active sulfur compounds are corrosive compounds foremost of Mercaptans which is having a formula of “RSH”. The typical temperature for the consummate “localized” reaction is approximately 80C and for the “sulfidation” process of corrosion it may be approached at about 230C [8]. The general reactions regarding the formations of the corrosion due to above processes have been given in the below.
S8(s) + 8 H2O (l) → 6 H2S (aq) + 2 H2SO4……… (2)
8Fe +S8→FeS…………….. (3)
According to the acidity results of both mineral oils Das Blend was consisted with higher amount of organic acids than the Murban. Organic acids are strong corrosive causing agent that fond as organic forms in most of crude oils which are defined under the general formula of “RCOOH” [2]. Acidity is an indicator regarding a mineral
oil that gives an idea about the presence of both organic acids and naphthenic acids in such mineral oil [9]. The general reactions of the cause of the metallic corrosion are given in the below.
Fe+2RCOOH→Fe(RCOO)2+H2…….……. (4)
FeS+2RCOOH→Fe(RCOO)2+H2S…………. (5)
Fe(COOR)2 + H2S →FeS + 2 RCOOH……… (6)
According to the salt contents in both crude oils Murban crude oil was composed in relatively higher amount of organic salts than Das Blend crude oil. The summation of the CaCl2, MgCl2 and NaCl contents of some crude oil is known as the total salt content in such crude oil. During increasing the temperature of the mineral oils such salts tend to be broken into HCl molecules approximately 1000C although such HCl molecules do not behave as corrosive compounds at this stage [7]. The general reaction for the initiation of such process is given in the below.
CaCl2 + H2O →CaO + 2HCl………..……. (7)
While reducing the temperature of the entire system some of HCl molecules tend to react with the water or moisture presence in the medium and produce hydrochloric acids and hydrogen sulfide. This is more prone process to the formation of the metallic corrosion as given in the below.
HCl(g)+H2O →HCl(aq)……………….… (8)
HCl + Fe→ FeCl2 + H2 …………….…… (9)
FeCl2 + H2S FeS + 2HCl………………….. (10)
FeS is the major corrosion compound that tend to be formed under this process and there might have a severe effect of hydrogen sulfide on the metallic corrosion although it is subtle to clarify because hydrogen sulfide is a gas and easy to evolve with having too short retention time period in the relevant mineral oil [2,4].
The average corrosion rates of each metal with respect to both Murban and Das Blend mineral oils have been interpreted in the Figure 2.
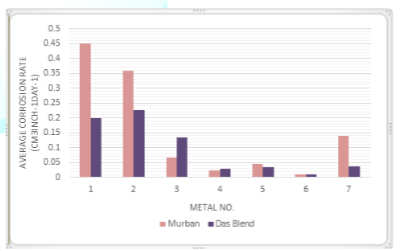
Figure 2: Average corrosion rates of metal coupons in both mineral oils.
The interpretation of the average corrosion rates of metal coupons is emphasized the relatively higher corrosion rates from carbon steels with respect to both Murban and Das Blend mineral oils, excessively lower corrosion rates form stainless steels with respect to both mineral oils and intermediate corrosion rates from Monel metal in both Murban and Das Blend mineral oils. The least corrosion rates were found form 321-MN: 1.4 304 - MN: 1.9 (Stainless Steel) in both Murban and Das Blend mineral oils. By referring the chemical compositions of stainless steels which were used in this experiment 321-MN: 1.4 304-MN: 1.9 (Stainless Steel) was composed ~18% of chromium and ~8% of nickel which is quite better chemical composition for the formation of the self-corrosive protection film on the metal surface because the minimum recommended chromium amount for that is ~12% and also sufficient amount of nickel [1,4,5]. The 410-MN: 1.7 420-MN: 1.7 (Stainless Steel) was composed ~12% of chromium and lack of nickel since it showed the maximum corrosion rates in both mineral oils among stainless steels. The 410-MN: 1.8 420-MN: 2.8 (Stainless Steel) was composed ~11% of chromium and ~0.2% of nickel also showed higher corrosion rates in both mineral oils than 321-MN: 1.4 304-MN: 1.9 (Stainless Steel). Therefore it can be concluded the necessity of the minimum recommended amount of chromium or more than that with sufficient amount of nickel to obtain the maximum benefits from the self-corrosive protection film against the corrosive environment.
When comparing the corrosion rates of metal coupons with respect to both mineral oils four types of metals showed higher corrosion rates in Murban since other three metal types were showing higher corrosion rates in Das Blend and entirely the distribution was an asymmetric distribution. In the explanation it can be emphasize the improper progress of the “localization” and “sulfidation” regarding the elemental sulfur and Mercaptans due to the requirements of higher temperatures because the current research was conducted under the normal environmental conditions. The effect of the salts on the metallic corrosion might be stronger than the effects of organic acids on the metallic corrosion according to the obtained results because Murban has higher salt content since Das Blend has higher acidity [2,8].
The variations of the corrosion rates of metal coupons with the exposure time period against both Murban and Das Blend mineral oils have been shown in order of Figure 3 and Figure 4.
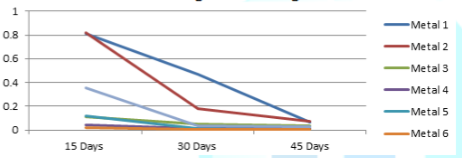
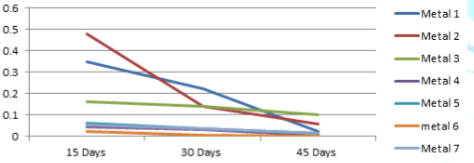
The variations of the corrosion rates of metal coupons with the exposure time period rudimentary there can be identified similar variations of the corrosion rate regarding most of metal types also prove the inversely proportional relationship between the corrosion rate and the exposure time period as mentioned in the formula of the weight loss method [10]. According to the microscopic analysis of the corroded surfaces of metal coupons some distinctive features have been highlighted in the Figure 5.
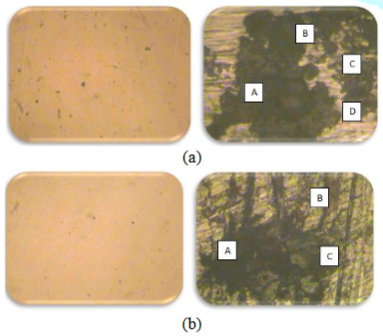
The analysis of such microscopic observations are based on the visible appearances of such corrosion compounds foremost the color and surface properties [1,3]. The general features of the visible appearances have been summarized in the Table 5.
· Ferrous Sulfides/ Copper Sulfides
· Ferrous Oxides
· Pitting Corrosion
· Corrosion Cracks

Table 5: Visible appearances of corrosion compounds.
Regarding the most of observations there were identified the formations of FeS according to the visible appearances of FeS. Especially regarding stainless steels some corrosion cracks and pitting corrosion phenomenon were observed apart from the formation of Fes. The formations of Fe2O3 were identified in seldom regarding some of carbon steels. There were observed the corrosion compounds on the surfaces of Monel metal which were much similar to the visible appearances of FeS [1,3]. By referring only such visible appearances foremost the blackish blue color it can be predicted as the CuS temporarily until an advanced compositional analysis of such chemical compounds.
The decayed ferrous concentrations of mineral oil samples which were interacted with carbon steels, stainless steels and the decayed copper concentrations of mineral oil samples which were interacted with Monel metal have been interpreted in order of Figure 6 and Figure 7.
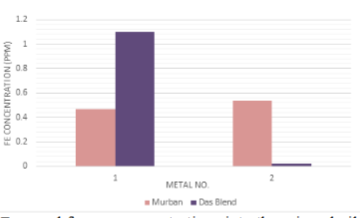
Figure 6: Decayed ferrous concentrations into the mineral oil samples.
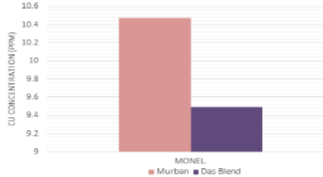
Figure 7: Decayed copper concentrations into mineral oil samples.
There were found higher decayed ferrous concentrations in crude oil samples with respect to the carbon steels (high) and carbon steels (medium) since other crude oil samples were free from decayed amounts of ferrous which were related with stainless steels. Also some significant decayed copper concentrations were found in crude oil samples which were related to the Monel metal. After formation of the corrosion compounds on the metal surface such compounds tend to be removed from the metal surface as soon as possible due to the attractive and repulsive forces between successive protons and electrons (Figure 7) [1,3,4]. Therefore, such ferrous and copper tend to be decayed into the mineral oils as corrosion compounds and the observed invisible weight loss during the determination of the corrosion rates of metal coupons can be explained easily with these observations and also these observations can be used as the confirmation evidences for the formation of metallic corrosion forever.
The deductions of the initial hardness of metal coupons due to the corrosion in both Murban and Das Blend mineral oils are shown in order of Figure 8 and Figure 9.
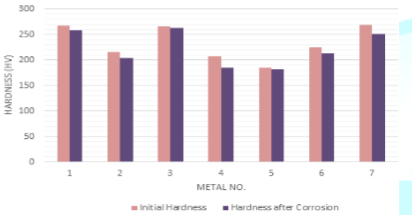
Figure 8: Deductions of the initial hardness of metal coupons in Murban.
The slight reductions of the initial hardness of metal coupons were observed by referring the obtained results for the variations of the initial hardness of metal coupons due to the corrosion. After the formation of corrosion compounds on the metal surfaces such compounds tend to remove form such metal surfaces due to the effects of the repulsive and attractive forces between successive electrons and protons while creating an instability status on the initial metallic surface [1,3]. Therefore, it can be emphasized the deductions of the hardness of metal coupons were happened due to the formation of metallic corrosion.
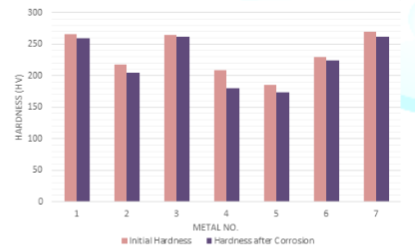
Figure 9: Deductions of the initial hardness of metal coupons in Das Blend.
Conclusion
Basically the obtained results interpreted the higher consistency in corrosive properties of Das Blend than Murban crude oil although the proper progress of every chemical process was not found at once especially the process of “sulfidation”, higher corrosive protection strength of stainless steels due to the naturally formed corrosive protection film, formations of the FeS as the corrosion compound on the metal coupons with some corrosion cracks and pitting corrosion in most of metals, decay of ferrous in high amount from carbon steels into both crude oils since carbon steels having higher corrosion rates, decay of copper in high amount from Monel metal into both crude oils and slight reduction in the initial hardness of each metal coupon due to the formation of the corrosion on the surfaces of metal coupons. There can be recommended some compositional analysis of the corrosion compounds that observed during the microscopic analysis by using some advanced analytical method such as the X-ray diffraction (XRD) for the future development of current research.
Acknowledgement
This task was supported and accommodated by the Ceylon Petroleum Cooperation, Sri Lanka, University of Moratuwa, Sri Lanka and Uva Wellassa University, Sri Lanka.
References
1. Khana OP. Materials Science and Metallurgy (2009) Dhanpet Rai and Sons, publication, India.
2. Alsahhaf TA, Elkilani A and Fahim MA. Fundamentals of Petroleum Refining (2010) Radarweg Press, Netherland.
3. Calister WD. An Introduction of Materials Science and Engineering (2003) John Wiley & Sons, USA.
4. Davis ME and Davis RJ. Fundamentals of Chemical Reaction Engineering (2003) McGraw-Hill, USA.
5. Singh R. Introduction to Basic Manufacturing Process and Engineering Workshop (2006) New Age International Publication, India.
6. Bolton W. Engineering Materials Technology (1994) B.H Newnes Limited, UK.
7. Ajimotokan HA, Badmos AY and Emmanuel EO. Corrosion in Petroleum (2009) Pipelines 36-40.
8. Speight JG. The Chemistry and Technology of Petroleum (1999) Marcel Dekker, USA.
9. Afaf GA. PhD. Thesis, University of Khartoum, 2007.
10. Okpokwasili GC and Oparaodu KO. Comparison of Percentage Weight Loss and Corrosion Rate Trends in Different Metal Coupons from two Soil Environments (2014) Int j Environ Bioremed Biodegrade 2: 243-249.
*Corresponding author:
Jagath K Premachandra, Department of Chemical and Process Engineering, University of Moratuwa, Katubedda, Sri Lanka, E-mail: bajagathp@gmail.com
Citation:
Aluvihara S and Premachandra JK. Essential stuffs for the cause of metallic corrosion in mineral oils (2018) Edelweiss Chem Sci J 1: 8-12
Keywords
Mineral oils, Corrosive properties, Ferrous metals, Weight loss, Corrosion rates


 PDF
PDF