Introduction
Bimatoprost, (7-[3,5-dihydroxy-2-(3-hydroxy-5-phenyl-pent-1-enyl)- cyclopentyl]-N-ethyl-hept-5-enamide), is antiglaucoma agent (ophthalmic); antihypertensive[1].
Bimatoprost is a prostaglandin analog/prodrug used topically (as eye drops) to control the progression of glaucoma and in the management of ocular hypertension. It reduces Intraocular Pressure (IOP) by increasing the outflow of aqueous fluid from the eyes. It has also been used and prescribed off-label to lengthen eyelashes [2-8].
A literature survey revealed few methods for determination of bimatoprost. Ultra Performance Liquid Chromatography (UPLC) with MS was reported for determination of the drug in presence its impurity (methyl ester) [9]. Another two HPLC methods were reported for determination of bimatoprost in bulk and ophthalmic solution [10,11], and HPLC-Tandem Mass Spectrometry Measurement of Bimatoprost, Latanoprost and Travoprost in Eyelash Enhancing Cosmetic Serums[12].
Also literature reveals there is no spectrofluorometric method was reported for bimatoprost. Spectrofluorimetric method proved to be more selective than normal UV-spectroscopy due to quantitation of substance at characteristic excitation and emission wavelengths. [13].
The objective of the presented work was to develop simple, economic, sensitive and rapid green analytical method for the quantitative determination of bimatoprost in drug substance, in ophthalmic dosage form, and in presence of interfering substance (benzalkolium chloride) by enhanced native spectrofluorimetric method. The fluorescence enhancement of a highly sensitive spectrofluorometric method is based on investigation of the fluorescence spectral behavior of bimatoprost in aqueous organized system β-CD. The host-guest interaction between bimatoprost and β-cyclodextrin inclusion complex was studied and the association constant was calculated.
Experimental
Apparatus
Cary Eclipse fluorescence spectrophotometric (USA) connected to IBM-PC computer and HP laser jet 1100 series printer. The emission of all samples was recorded against a solvent blank in 1 cm quartz cuvettes and scanning at the following parameters:Band width = 1.5 nm, speed = 1200 nm/min, Data Interval = normal (1nm), Smoothing = high, Jenway Digital pH meter model 8417 was used for adjusting the pH. Shimadzu Model RF-160, UV/VIS spectrophotometer was used.
Samples
Pure sample: Bimatoprost was kindly supplied by Chemipharm Co., Egypt. Its purity was found to be 99.80% according to the manufacturer method [14].
Market samples: Lumigen™ ophthalmic solution was labeled to contain 0.03%, El -Sofikopharm Co., Egypt; Batch No. 85543 was purchased from the market.
Chemicals: All chemicals used were of analytical reagent grade and solvents were of HPLC grade.
Acetonitrile, methanol, ethanol, and acetone (Macron fine chemicals, Poland), sodium hydroxide (Merck, Darmstadt, Germany), β- Cyclodextrin Sigma Aldrich (Germany), Benzalkolium chloride Sigma Aldrich(Germany), Double distilled water was used throughout all experiments after filtration through a 0.47 μm membrane filter (Alltech Associates, USA).
Standard solutions
A stock standard solution of bimatoprost (0.1 mg/mL) was prepared by dissolving 10.00 mg of bimatoprost in water in 100 mL volumetric flask and the volume was completed to the mark with the same solvent. Working standard solution (10 μg/mL) was prepared by transferring 10 mL of stock solution into a 100 mL volumetric flask and completed to the mark with water.
Procedures
Construction of the calibration graph
a. In absence of 1% β-CD: Aliquots equivalent to 250- 2500 ng/mL of the working standard solution is transferred into a series of 10 mL volumetric flasks completed to the mark with water to give a final concentration range of 25.00-250.00 ng/mL.
In presence of 1%(w/v) β-CD: Aliquots equivalent to 50 – 500.0 ng/mL of the working standard solution were transferred into a series of 10 mL volumetric flasks by graduated micropipette followed by 1.5 mL of 1% (w/v) β-CD and then completed to the mark with water to give a final concentration range of 5 – 50.00 ng/mL.
The fluorescence intensity was measured versus the concentrations of the drug (ng/mL) at λem 285 nm after excitation at λex 217 nm. The calibration graphs were plotted. Then the regression equations were computed for the drug in absence and presence of β-CD respectively.
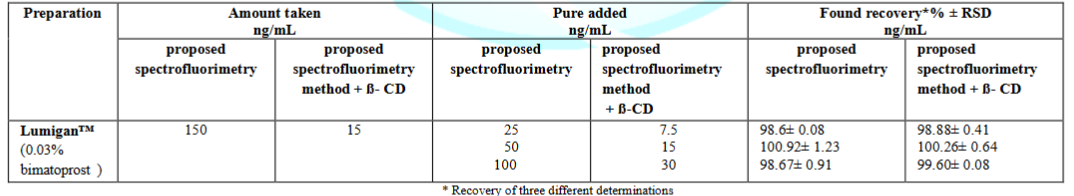
Application: The proposed methods were successfully applied for the determination of bimatoprost in its pharmaceutical dosage form Lumigan™ (Bimatoprost ophthalmic solution, labeled to contain 0.03%). A Stock solution was prepared by mixing the content of three bottles (9 mL) in a stopper conical flask. Each milliliter was equivalent to 0.03 mg of bimatoprost.
In absence of 1% β-CD: An accurately measured volume of ophthalmic solution equivalent to 3 µg bimatoprost was transferred to volumetric flask of 10 mL capacity. The volume was completed with water. Then transfer 5.0 mL of dosage stock solution to volumetric flask of 10 mL capacity and completed with water to obtain solution equivalent to 150 ng of bimatoprost.
In presence of 1% β-CD: An accurately measured volume of ophthalmic solution equivalent to 3.0 µg bimatoprost was transferred to volumetric flask of 10 mL capacity. The volume was completed with water. Then transfer 0.5 mL of dosage stock solution to volumetric flask of 10 mL capacity, followed by 1.5 mL of 1% (w/v) β-CD and completed the volume with water to obtain solution equivalent to 15 ng of bimatoprost. The nominal content of the eye drop was determined using either the calibration graph or the corresponding regression equation.
Specificity: Accurately
transfers 10 mg of Benzalkolium
Chloride to 10 mL volumetric flask and complete with water to the mark to
obtain a final concentration of 1mg/mL. A further dilution step was made to
fall in the working range of each developed method. Then the recommended
procedure mentioned under 2.4.1 was proceeded.
Results and discussion
Spectral characterization
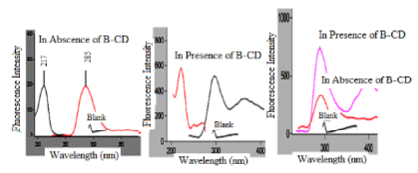
The native fluorescence of bimatoprost was measured at λem 285 nm after excitation at λex 217 nm in water. Bimatoprost is characterized by having a native fluorescence due to its fused aromatic rings and extended conjugated structure. Emission and excitation spectra of bimatoprost were given in Figure 1. Fluorescence spectra of bimatoprost in absence and presence of β-CD were investigated (Fig. 1). Maximum emission wavelength of bimatoprost and bimatoprost/ β -CD complex was observed at 285 nm. The results suggest that a stable complex was formed between β-CD and bimatoprost. The quantum yield [QY] was calculated in absence and presence of β-CD and it is found to be increased from 0.26 to 0.31. Quantum yield was calculated according the equation [15]: The enhancement of native fluorescence intensity in aqueous organized media is due to change in viscosity, polarity and binding capacity [16,17].
QY = Үs. Fu / Fs. As /Au, QY = Quantum yield, As = Absorbance of standard, Au = Absorbance of unknown, Ys = Quantum yield of standard, FU = Integrated emission of unknown, Fs = Integrated emission of standard.
Optimization of reaction conditions
Different experimental parameters affecting the native fluorescence intensity of the drug and its stability were carefully studied and optimized.
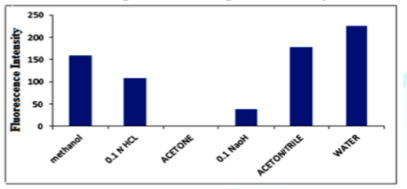
Influence of diluting solvents: The effect of different diluting solvents on FI of bimatoprost was investigated upon dilution with different solvents including methanol, 0.1 M HCl, acetone, 0.1 M NaOH, acetonitrile and distilled water. No fluorescence was observed with acetone. Each of diluted aqueous acid, aqueous alkali, acetonitrile and methanol decrease the intensity of fluorescence of bimatoprost compared to water. Water gave the highest fluorescence intensities compared with the other solvents as shown in Figure 2. Thus, water was chosen as the diluting solvent throughout the study.
Effect of surfactants: The effect of 1.0 (w/v) aqueous solution of several types of surfactants namely, β-Cyclodextrin, tween 20, tween 40, and cetyltrimethyl ammonium bromide was investigated. The relative fluorescence intensity was studied by adding 1 mL of each surfactant solution to the drug solution in water. The relative fluorescence intensity for each solution was measured within 30 min each against the appropriate blank as presented in Figure 3.

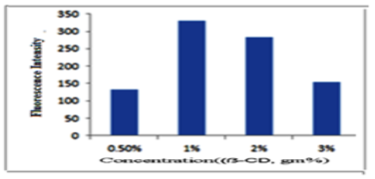
Influence of different concentrations of 1% (w/v) ẞ-CD: The fluorescence intensity of bimatoprost in different concentrations of β-CD from 0.5 to 3.0 % (w/v) was investigated. The results revealed that the highest intensity was observed at concentration of 1 % (w/v) β-CD. The results are shown in Figure 4.
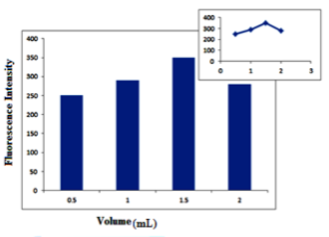
Influence of different volumes of 1.0% (w/v) β-CD: The effect of different volumes of β-CD, 1% (w/v) from 0.5 – 3.0 mL was investigated. It was found that 1.5 mL is the best volume, as it gave the highest FI as shown in Figure 5.
Determination of complex-ratio and formation constant
Standard fluorescence spectroscopy analyzes the variation of a spectroscopic property (quantum yield, spectral shift, lifetime, or anisotropy) of a fluorescent guest or host due to the complexation. A significant variation of any of these parameters requires an intimate participation of the fluorophore in the complexation process. The formation of a host-guest inclusion complex of bimatoprost with (β-CD) in aqueous organized solution has been characterized by fluorimetry. The nature of the host-guest inclusion complex between bimatoprost and β-CD has been elucidated. The experimental results confirmed the existence of 1:1 inclusion complex. The binding constants describing the extent of formation of the complex have been determined, using modified Benesi-Hildebrand plots [18,19]. The schematic presentation of the inclusion is presented in scheme 1.
The ratio of complex, and formation constant were calculated from the modified Benesi-Hildebrand equation, 1/ (F-Fo) = 1/(Kk[P]o[CD]o) + 1/(Kq[P]o)
Where F, Fo represent the fluorescence intensity of bimatoprost in absence and presence of ẞ-CD, respectively, K is the formation constant, and [p] is constant. The reciprocal plots of 1/ (F-Fo) versus 1/[CD] showed good linearity ( Figure 6), indicating that the inclusion complex has a stoichiometry of 1:1. The value of k was found to be 137.54 M-1.
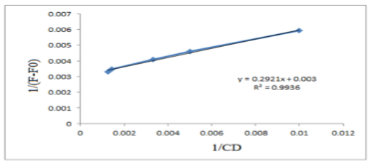
Figure 6: Incluion complex of bimatoprost – β-CD (1:1)
Method validation
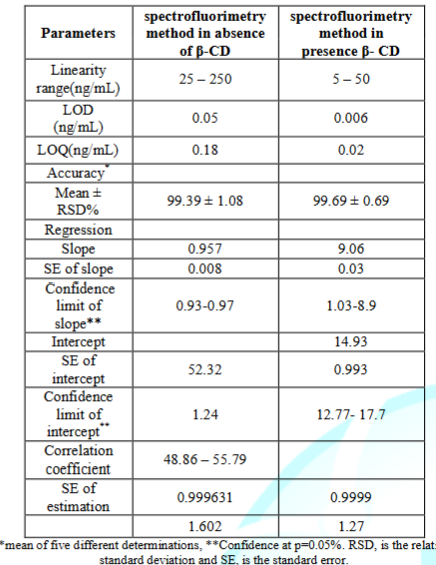
The validity of the proposed method was assessed by studying the following parameters: linearity, range, LOD, LOQ, accuracy, precision, robustness and specificity, according to ICH guidelines (20) and USP (21), the results were presented in Table 1.
Linearity and range
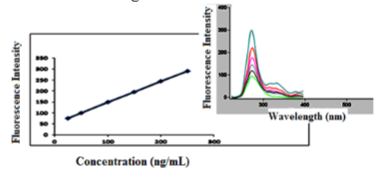
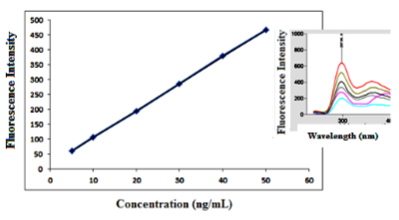
There was linear relationship between bimatoprost concentration and the native fluorescence obtained over the concentration range of (25.00-250.00 ng/mL), (5.00-50.00 ng/mL) in absence and presence of 1.5 mL of 1% β - CD, respectively as shown in Figure (7,8). The results showed good linearity with regression parameters calculated according to ICH guidelines as in Table 1.
The regression equations were computed and found to be as the following:
FI = 0.9562 C + 52.529 R2 = 0.9997 in absence of 1% β-CD
FI = 9.066 C + 14.94 R2 = 0.9999 in presence of 1% β-CD
Where: FI is the fluorescence intensity, C is the concentration in ng/mL.
The high values of correlation coefficient (R2) and low values of Standard Deviation (SD), Standard Error (SE), and Relative Standard Deviation (RSD) showed the assemblage of the points around the calibration graph and proved the linearity of the method over the specified concentration range as shown in Table 1.
Limit of detection (LOD) and limit of quantification (LOQ)
LOD and LOQ were calculated according to the following equations as
specified by ICH guidelines and the results are summarized in Table 1.
LOD = 3.3 σ / S
LOQ = 10 σ / S
Where σ is the standard deviation of the response and S is the slope of linearity.
Accuracy
To prove the accuracy of the proposed methods, the results of the assay of the drug substance was assessed by the proposed spectrofluorimetric method and compared with those obtained using manufacture HPLC methods [14].
Statistical comparison of the results obtained by the proposed method and those obtained by the manufacture method using mean recoveries, Student’s t-test and variance ratio F-test revealed no significant difference between the two methods regarding accuracy and precision as shown in Table 2, indicating high accuracy and precision of the proposed methods [20, 21].
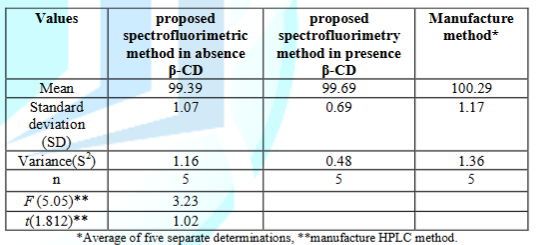
Precision (repeatability and intermediate precision)
The intra- and inter-day precision were assessed by assaying freshly prepared solutions in triplicate on the same day and on three different days, respectively using the proposed methods. The low RSD of the repeatability (intra-day) and intermediate precision (inter-day) of the results obtained by means of the proposed methods indicate a high precision of these methods and proved to be suitable for quality control of bimatoprost as shown in Table 3.
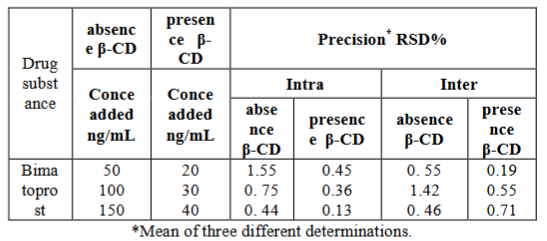
Specificity
The specificity of the proposed spectrofluorimetric methods were proven by its ability to determine bimatoprost in pharmaceutical reparation without interference from Benzalkolium Chloride that commonly present in the matrix as represent in Table 4.
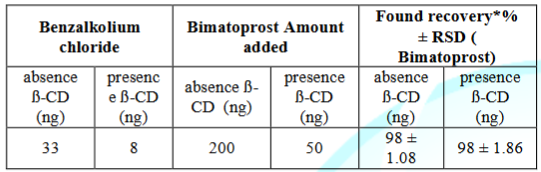
Stability of reference and working solutions
Solutions of the drug were stable for one month when kept in the refrigerator. No change in fluorescence intensity appeared throughout the whole validation procedures.
Application
The proposed methods were successfully applied for the determination of bimatoprost in its pharmaceutical dosage form, Lumigan™ (Bimatoprost ophthalmic solution was labeled to contain 0.03%).
The proposed methods after sample preparation discussed before under section 2.4.1.was successfully used to quantify bimatoprost in pharmaceutical dosage form as shown in Table 5.
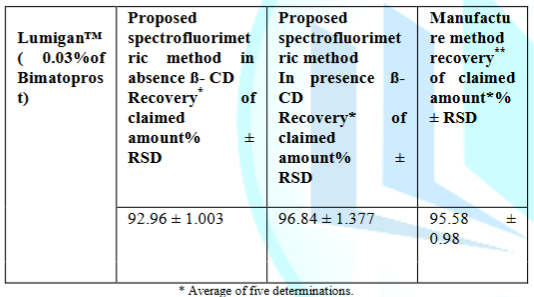
Standard addition technique was used to assess the matrix effect of the solution additives and its contribution in the deviation of the results obtained by the proposed methods. The obtained results revealed no significant matrix effect as shown in Table 6.
Conclusion
In the present work emission spectra of bimatoprost in absence and presence of β-CD were investigated. The fluorscence spectroscopy of the host – guest interaction between bimatoprost and β-CD shows 1:1 inclusion complex, with an association constant of 137.54 M-1. The quantum yield was 0.26 and 0.31 in absence and present of β-CD. To the best of our knowledge, no report of fluorimetric methods were studied before for determination of bimatoprost. The advantages of the methods are cheapness, ecofriendly, rapid and specific and can be used for the routine quality control of drug in drug substance and pharmaceutical eye drop with no potential interferences from excipients. The results showed that the quantity of drug substance in drug product was in a good agreement with given labeled quantity. The proposed spectrofluorimetric method is characterized by being, simple, available, and more sensitive and having shorter time of analysis when compared to tedious chromatographic methods. The suggested methods are greener than the reported ones with good validation parameters and hence they can be used for routine analysis of the studied drug without harming the environment.
Acknowledgements
First, great thanks for GOD who offers to me all responsibilities to get that work. Great thanks for all my professors who they are contributing with me in that paper. Great thanks for my faculty and NODCAR, and my lovely home EYGPT.
Reference
1. Sean CS. Martindale: The Complete Drug Referenc. 36th (2014) Pharmaceutical Press, London-Chicago 2784.
2. Chen M, Cheng C, Chen Y, Chou C and Hsu W. Effects of bimatoprost 0.03% on ocular hemodynamics in normal tension glaucoma (2006) J Ocul Pharmacol Ther 22: 188–193.
3. Laurence LB, John SL and Parker KL. Goodman & Gilman’s The Pharmacological Basis of therapeutics (2006) 11th McGraw Hill Professional.
4. Kruse P, Rieck P, Sherif Z and Liekfeld A. Cystoid macular edema in a pseudophakic patient after several glaucoma procedures. Is local therapy with bimatoprost the reason? (2006) Klinische Monatsblätter für Augenheilkunde 223: 534–537.
5. Steinhäuser S. Decreased high-density lipoprotein serum levels associated with topical bimatoprost therapy (2006) Optometry 77: 177–179.
6. Park J, Cho HK and Moon JI. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost (2011) Japanese Ophthalmol Soc 55: 22–27.
7. Jayaprakasam A and Ghazi-Nouri S. Periorbital fat atrophy - an unfamiliar side effect of prostaglandin analogues (2007) Orbit 29: 357–359.
8. Filippopoulos T, Paula JS, Torun N, Hatton MP, Pasquale LR, et al. Periorbital changes associated with topical bimatoprost (2008) Ophthalmol Plastic Reconstruct Surg 24: 302–307.
9. Murali KP, Rao BT, Bujagendra RM, Rao CN, Kishore KR, et al. Determination of a novel impurity by LC-MASS and chromatographic separation of bimatoprost, isomers and their impurities by UPLC (2011) J Pharm Res 4: 2381-2383.
10. Krishna M, Rao T, Raju B, Narasimha Rao, Kumar K. Development and Validation of RP-HPLC Method for Estimation of Bimatoprost in Pharmaceutical Dosage Forms (2011) J Pharmacy Research 4: 3733.
11. Suresh SK, Natraj K, Asadulla K, Kalyan BK and Venkateshwara JR. Development and Validation of RP-HPLC Method for Estimation of Bimatoprost in Pharmaceutical Dosage Forms (2009) J Pharmacy Research 4: 3733-3734.
12. Marchei E, Daniela De Orsi , Guarino C, Rotolo M C, and Pichini S. High Performance Liquid Chromatography Tandem Mass Spectrometry Measurement of Bimatoprost, Latanoprost and Travoprost in Eyelash Enhancing Cosmetic Serums (2016) Cosmetics 3: 1-8
13. Chen GZ, Huang XZ, Xu JG, Zheng ZZ and Wang ZB. The Methods of Fluorescence Analysis (1990) Science Press, Beijing, 2: 112.
14. Chemipharm Company Manufacture Method.
15. Skillman JB. Quantum yield variation across the three pathways of photosynthesis: not yet out of the dark (2008) J Exp Bot 59: 1661-1647.
16. Brouwer AM. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report) (2011) Pure Appl Chem 83: 2213–2228.
17. Sharma BK. Instrumental Methods of Chemical Analysis (2002) 21st Edition, Goel Publishing House, Meerut, 360-373.
18. Benesi H and Hildebrand JA. Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons (1949) J Am Chem Soc 71: 2703–2707.
19. Anslyn Eric. Modern Physical Organic chemistry (2006) University Science Books, United States 1095.
20. ICH Q2B Validation of analytical procedure: methodology, In IFPMA (ed) (1996) International Conferences on Harmonization, Geneva
21. United States Pharmacopeia USP 39 National Formulary 34 (39th Ed) Rockville, United States: United States Pharmacopeial Convention INC. 2018.
*Corresponding author:
Maha A Elabd, Department of Pharmaceutical Chemistry, National Organization for Drug Control and Research, 6 Abou-Hazem st, Giza, Egypt, E-mail: mahaelabd@hotmail.com
Citation:
Walash MI, Toubar S, AbouEl-Alamin MM, Elabd MA and Salama NN. Spectrofluorimetric study on inclusion interaction of β- cyclodextrin with bimatoprost: challenging to green analytical applications (2018) Edelweiss Chem Sci J 1: 2-8


 PDF
PDF