Research Article :
Wendy Zhao,
Xinyi Mei, Zheng Yue and Braja K Mandal A new class of lithium-ion conducting Solid
Polymer Electrolytes (SPEs) has been derived from oligomeric Polyethylene Oxide
(PEO)-grafted Cross-linked Polystyrene (XPS) microspheres containing one or two
lithium sulfonamide moieties. The SPE containing Li:O mole ratio of 1:8
displayed excellent ionic conductivity (in excess of 10-4S/cm at 25ºC)
and good electrochemical stability (4.3 volts versus Li/Li+).
Thermal properties of these SPEs have also been investigated with Differential
Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA). These new SPEs
possess amorphous character with a glass Transition Temperature (Tg)
around 135ºC, and no significant thermal decomposition until 420ºC.
Synthesis and characterization including surface morphologies of these SPEs are
described. The development of high energy density batteries
with good safety and reliability has been an active area of research for the
past three decades [1-5]. Advances in portable electronic devices, such as cell
phones and laptops, have created a demand for smaller, lighter, yet more
powerful energy sources. In particular, lithium-ion
(Li-ion) batteries have received the most attention, due
to the high redox potential of lithium and outstanding cycling stability [6,7].
Significant research has been focused on the development of electrolyte
materials, which transport lithium ions between the electrodes. Although polar
aprotic liquid electrolytes and gel polymer electrolytes containing 50-80%
organic solvent can be used as the media for the transport of lithium ions, the
requirement for bulky enclosures and the reported safety-related shortcomings
make them less attractive for future applications [8-10]. Accordingly, several
attempts have been made to develop SPEs that allow the use of complex shapes,
greater ease of fabrication, free of leakage and much lower flammability. In the past decades, numerous research studies have
been concentrated on the PEO-based SPEs. PEO with C-O, C-C and C-H on the
backbone has a good combination of chemical and electrochemical properties. PEO
contains both crystalline and amorphous regions, and is very flexible (low glass
transition temperature, Tg=-61ºC). The most positive
attribute of PEO-based SPEs is their high dissolution of lithium salts. The
lithium ion conduction takes place in the amorphous phases of PEO via
diffusion, and the lithium ion migration associated with segmental mobility of
the PEO chains. The major drawback of PEO-based SPEs is their poor room
temperature ionic conductivity due to the high crystallinity of PEO-lithium
salt complex. Thus far, no SPE is known that efficiently transports lithium
ions at conductivities in excess of 10-3Scm-1 at 25ºC,
which is considered commercially viable [11-14]. Recently, polymer microspheres, such as XPS beads,
have attracted a great deal of interest in developing new materials due to
their special particle morphologies and characteristics, such as large specific
surface area, remarkable aggregation effects, and surface reaction capability
[15-18]. Thus, XPS beads can act as building blocks in construction of new
micro-structured hybrid systems, owing to their ease of chemical modification
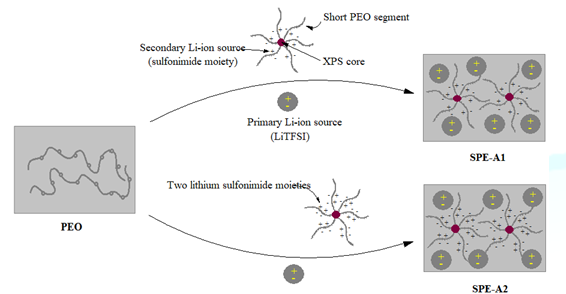
[19-23]. In this study, we have synthesized and examined two SPEs (SPE-A1 and
SPE-A2), in which XPS beads are grafted with oligomeric PEO chains through a
sulfonamide moiety containing one or two lithium ions [24-26]. The motivation
for such a molecular design originated from the fact that lithium ions can be
transported easily in this hybrid system by introducing a large number of
highly flexible pendant PEO oligomeric chains on the outer surface of XPS beads
(Figure 1). Such a structural
modification would provide ideal Li+ conducting pathways at the XPS
particle surface, leading to superior ionic transport [27,28]. The presence of
numerous oligoether short chains on the peripheral surface of the XPS will
allow faster ion mobility compared with a linear coil having the same molecular
weight. This is because, in a given polymer, the chain end is more flexible
than the chain middle [29]. Moreover, covalently-linked lithium sulfonamide
moieties provide a secondary source of lithium ions, leading to superior ionic
transport properties. Additionally, the thermal stability of the SPE is
expected to improve due to the presence of XPS beads in the SPE. Figure1:Schematic of the proposed SPEs Materials Commercially available chlorosulfonated
XPS,
XPS-SO2Cl, with diameters near 128µ, was provided by Biotage LLC, NC.
3-Nitrobenzenesulfonyl
chloride was obtained from Sigma-Aldrich Chemicals, MO. Zinc
dust, commercial grade (total zinc 98.5%), was obtained from U.S. Zinc®,
TX, and used as received. Di-ammonium hydrogen phosphite was purchased from MP
Biomedicals LLC, OH. Lithium bis(trifluoromethane)Sulfonimide (LiTFSI) was
obtained from 3M, MN. The monoamine-terminated oligomeric PEOs, Jeffamine®
M-600 (Mw 600, PO/EO 9/1) and M-1000 (Mw 1,000, PO/EO 3/19), were received as
gift samples from Huntsman Corporation, TX. Synthesis
of XPS-Li-PEO 2g of XPS-SO2Cl, 9g of Jeffamine®
M-1,000 (or 6g of Jeffamine® M-600) and 25 mL of dry THF were placed
in a three-necked round bottom flask (50mL) connected with a reflux condenser. After
5 min, 2 mL of triethylamine, diluted with 10mL of dry THF, was added slowly to
the mixture under stirring through a dropping funnel. The reaction was
conducted under argon in an ice water bath for the initial 2 hours and
subsequently stirred at room temperatures overnight. The sulfonamide product
was filtered using a sintered funnel and washed with 20mL of water, 20mL of
methanol, and then 10mL of acetone. The product was dried under vacuum for 24
hours prior to the lithiation
reaction, which was performed by stirring overnight the
product with an excess of 1M LiOH solution. XPS-Li-PEO was isolated by
filtration followed by the aforementioned sequence of the washing protocol. Synthesis
of XPS-Li2-PEO Nitro
Derivative:
2.5g (0.011mol) of 3-nitrobenzenesulfonyl chloride, 15g (0.015mol) of Jeffamine®
M-1,000 (or 8.8g of Jeffamine® M-600) and 50mL of methylene chloride
were mixed in a 100mL three-necked round bottom flask, which was set in an ice
bath and equipped with a dropping funnel. 1mL of triethylamine diluted with 10mL
of methylene chloride was added drop-wise to the mixture. The reaction was
continued overnight under argon at room temperature. After the reaction, HNEt3Cl
was filtered off and the filtrate was transferred into a 100mL flask, and 50mL
of ether was added to yield more HNEt3Cl, which was filtered off. The
filtrate was transferred to a flask with 50 mL of hexane and kept in the
freezer overnight, with the formation of two layers. Excess Jeffamine®
was removed by decanting the hexane layer. This freezing-decantation procedure
was repeated three times to obtain the pure nitro derivative. Yield: 6.97g
(62%). Amino
Derivative:
The aforementioned nitro compound, 2g (2.55 mmol), and commercial grade zinc
dust, 0.2g (3.05mmol), in methanol (2mL) were stirred with 0.68g (5.07mmol) of
di-ammonium hydrogen phosphite at room temperature. After 45 min, reaction was
complete, monitored by TLC (silica plate chloroform/methanol=90/10, v/v). The
mixture was filtered to remove residual zinc dust. The organic layer was
evaporated and the residue dissolved in 10mL of methylene chloride and washed
with saturated sodium chloride solution to remove ammonium formate. The organic
layer was evaporated in a rotovap and the product dried under high vacuum to
obtain the amino compound. Yield: 1.52g (76%). 1H-NMR (300MHz, CDCl3):
δ (ppm): 1.09 (polyether CH3), 3.20-3.40 (polyether CH2),
3.50 (polyether CH), 3.45 (polyether CH3), 7.50-8.50 (aromatic CH),
6.28 (aromatic NH2) and 6.45 (sulfonamide NH). XPS-Li2-PEO: The
synthesis of the bis-sulfonamide
derivative and the subsequent lithiation reaction were
performed according to the procedure described earlier for XPS-Li-PEO, using 2g
of XPS-SO2Cl and 9g of the aforementioned amino derivative. Preparation
of SPE Film Ten different polymer electrolyte films with varied
compositions were prepared (Table 1).
The SPE-A was based on Jeffamine® M-1000. SPE-B was based on
Jeffamine® M-600; A1 and B1 contain a single lithium sulfonamide
group per repeat unit, while A2 and B2 contain two lithium sulfonamide groups
per repeat unit. The primary source of lithium ions was attributed to LiTFSI. The
ratios of Li:O (lithium ion per ethylene oxide moieties) were varied from 1:6
to 1:12 (Table 1). Only 3.5% by weight of PEO (Mw=4,000,000) from Sigma-Aldrich was
added to each formulation to obtain free-standing thin films (~0.150mm). The traditional solvent casting method was employed
to cast thin films [30-32]. The components for each sample were dissolved in
acetonitrile in a round bottom flask, then poured into a beaker and kept at 60ºC
under vacuum to remove solvent. The resulting film was placed between two PTFE
coated sheets and hot-pressed (100ºC, 500 psi, 30 seconds). The film
thickness was controlled using two thin steel plates as spacers. Table1:Compositions of SPE films. Ionic
Conductivity The ionic conductivities of the electrolytes were
measured using Electrochemical
Impedance Spectroscopy (EIS). The SPE films were cut into a
circle and placed between two steel electrodes and subjected to an impedance
analyzer [30-32]. Ionic conductivities were measured with all ten SPE films at
temperatures ranging from 25ºC to 70ºC. A Solartron®
model SI-1287 electrochemical interface coupled to a Solartron®
model-1260 frequency response analyzer was used to obtain the EIS measurements.
The bulk resistance was determined from the high frequency intercept of the
real axis of the complex impedance plot. The conductivity was calculated using
the equation σ=I/RbA, where I is the thickness, A is the area of the testing
films, and Rb is the measured bulk resistance. Cyclic
Voltammetry The electrochemical stability of a representative
sample SPE-A2 was measured by Cyclic
Voltammetry (CV) using a Hohsen electrochemical
test cell, with a stainless steel working electrode and lithium film
counter/reference electrode, while the solid polymer electrolyte was placed
between working and counter/reference electrodes [30-32]. All the preparation
steps were carried out in side an Argon filled glove box. CV is performed with
a computer controlled Solartron® 1287 Electrochemical Interface,
employing the CorrwareTM software. The experiments were performed by
applying a voltage sweep from 2V to 5V at a scan rate of 10 mV/s on cells that
are thermally equilibrated at 25ºC for at least 1 hour. Differential
Scanning Calorimetry Measurements The Differential
Scanning Calorimetry (DSC) measurement of representative
sample, SPE-A2/1:8 was conducted and compared with a reference (control-2)
polymer electrolyte, using Seiko DSC 220C. The scanning was performed from 25ºC
to 200ºC to observe the changes in thermal properties of the polymer
electrolytes. DSC scanning cycles included equilibration at 25ºC,
ramp 10ºC per minute to 200ºC, mark end of cycle 0,
isothermal for 5.0 minutes, ramp 10ºC per minute to 25ºC,
mark end of cycle 0 [30,31]. Thermogravimetric
Analysis Measurements The thermal stability of representative sample,
SPE-A2/1:8 and the reference sample, control-2 was measured by TGA, using
TG/DTA 6200 system. Sample was heated in the analyzer from 25ºC to 500ºC at a
heating rate of 10ºC per minute under inert atmosphere [30-32]. Film
Images by Fluorescence Microscope Optical images of the best polymer electrolyte film
(SPE-A2/1:8) were obtained by using an Olympus
Fluorescence/ Differential
Interference Contrast (DIC) Analytical Digital Microscope and
ColorView soft imaging system with objectives of UMPlanFI 5X/0.15, UMPlanFI
20X/0.46 and UMPlanFI 50X/0.80 and filters, U-AN 360-3 and U-P03. Synthesis
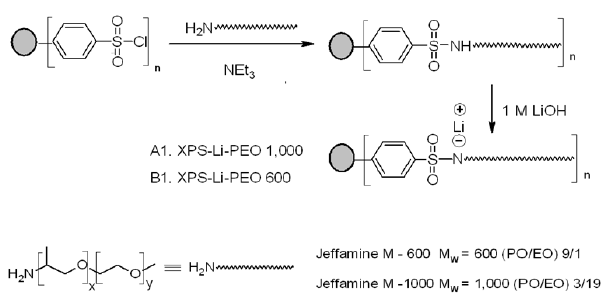
of PEO-Grafted XPS (XPS-Lix-PEO) Figure
2
depicts the general methodology for the preparation of XPS-Li-PEO in two steps
starting from commercially available XPS-SO2Cl. The first step
involves reaction with an excess polyetheramines (Jeffamines®, Mw
600 or 1000) in the presence of triethylamine to produce sulfonamide
derivatives. In the second step, the XPS-Li-PEO derivatives were obtained by
treating sulfonamide derivatives with LiOH. The structures of both the
intermediate and the final product were confirmed by the FTIR spectroscopy (Fourier-Transform
Infrared Spectroscopy). The spectrum of the amide compound showed these
signature characteristic peaks: 1368 and 1165 cm-1 (sulfonyl), 3324,
1601, and 758 cm-1 (amide N-H), 2983 and 1470, cm-1
(aliphatic C-H) and 1030 and 608 cm-1 (C-O-C). The signature peaks
for XPS-Li-PEO appeared at: 1377 and 1172 cm-1 (sulfonyl), 3003 and
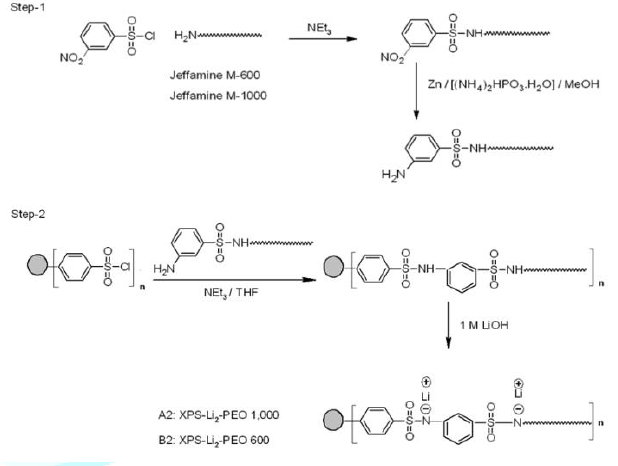
1450 cm-1 (aliphatic C-H), 1082 and 578 cm-1 (C-O-C). For the preparation of PEO-Li2-XPS (Figure 3), first the polyetheramines
(Jeffamines®, Mw 600 or 1000) were treated with
3-nitrobenzenesulfonyl chloride. The resulting nitro derivative was reduced to
the corresponding amino compound using a mild procedure involving zinc-dust and
di-ammonium hydrogen phosphite [33,34]. Once the amino compound was obtained, then
PEO-Li2-XPS was conveniently prepared following the strategy
established for PEO-Li-XPS. The structures of the intermediates and the final
product were confirmed by the FTIR spectroscopy. The IR spectrum (Infrared
Spectrum) of the polyether tethered 3-nitro sulfonamide compound showed the
signature peaks at 1535 and 1380 cm-1 for aromatic nitro group. The
spectrum of the polyether tethered 3-amino sulfonamide compound showed a
doublet for the primary aromatic amine at 3459 and 3382 cm-1. Other
characteristic peaks include 1375 and 1117 cm-1 (sulfonyl), 1605 and
782 cm-1 (amide N-H), 2967 and 1463 cm-1 (aliphatic C-H)
and 1256 and 523 cm-1 (C-O-C). The structure of the amino compound
was also confirmed by 1H-NMR (300 MHz, CDCl3). Figure2:Synthesis of XPS-Li-PEO. Figure3:Synthesis of XPS-Li2-PEO. Characterization
of SPEs Ionic
conductivity: Ionic conductivities were measured with all SPE
complexes containing LiTFSI (Li/O ratio: 1/12-1/6) and 2-5 wt.% PEO (Mw=400,000)
to improve the flexibility of the free-standing thin SPE films (0.12-0.15mm).
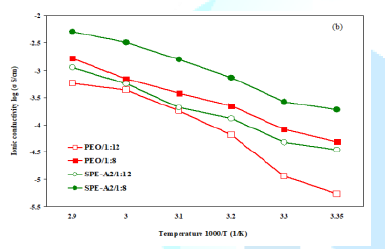
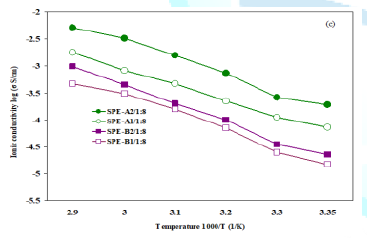
The ionic conductivities of XPS-Lix-PEO SPE, along with a standard
PEO SPE (control-1 and 2 in Table 1), are shown as Arrhenius plots in Figure 4a-Figure 4d. Compared with the
standard PEO SPE, the new SPEs provided higher ionic conductivity in the
temperatures range of 25ºC to 70ºC (Figure 4a, Figure 4b). The ionic conductivity increases with the
increase of the number of lithium sulfonamide groups and the polyetheramine
segment with more ethylene oxide repeat units (such as Jeffamine®
M-1000 (PO/EO 3/19)). The SPE with Jeffamine® M-600 (PO/EO 9/1)
displayed lower ionic conductivity. The highest ionic conductivity was obtained
with the film assigned as SPE-A2/1:8: 1.87 x 10-4S/cm at 25ºC
and 5.05 x 10-3S/cm at 70ºC. This SPE was synthesized
with Jeffamine® M-1000 in conjunction with two sulfonamide groups (Figure 4c). Figure
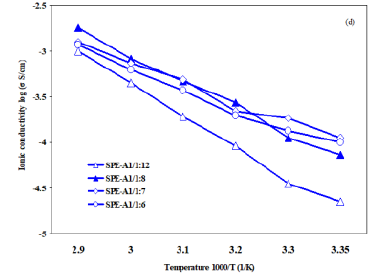
4d
shows the ionic conductivity of the SPE-A1 with 4 different loading levels of
LiTFSI in the Li:O ratios of 1:12, 1:8, 1:7 and 1:6. Results indicate that the
ionic conductivity of SPE with Li:O ratio of 1:8 is much higher than 1:12. However,
this trend was not followed by continually increasing the amount of the salt in
the samples with Li:O ratio of 1:7 and 1:6. In fact, after the conductivity
reaches to the maximum point at the composition with Li:O ratio of 1:8, the
addition of excess lithium salt does not continue to increase the ionic
conductivity. This has been explained that as the salt is increased above an
optimum concentration, the distance between ions becomes significant smaller,
the ions become closer to one another and tend to associate to form ion
aggregates or ion clusters in the matrix; which could reduce the number of
free-ions and restrict the chain segment mobility, leading to low ionic
conductivity [35-37]. Figure4a:Ionic conductivities; New SPEs vs. Control. Figure4b:SPE-A2 (1000 M-Li2-XPS) vs. Control. Figure4d:Evaluation of LiTFSI loading level on SPE-A1 Electrochemical
stability:
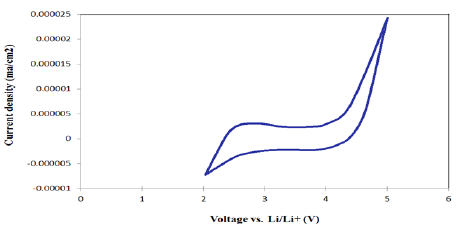
Electrochemical
Stability Window (ESW) is one of the most important
parameters for the electrolytes used in electrochemical cells. The ESW of the
electrolyte, defined as the voltage range between which the SPE is neither
oxidized nor reduced, is usually measured by the CV experiment. As shown in Figure 5, the PEO-Li2-XPS
SPE displayed good electrochemical stability. The representative film comprised
of SPE-A2/1:8, was subjected to an applied field of 2 to 5 volts versus Li/Li+.
No oxidation or reduction peak in the voltage window of 2.5 to 4.3 volts was
observed. The oxidation peak which is ascribed to the breakdown of PEO-Li2-XPS
system was not appeared until 4.3 Volts versus Li/Li+ [38,39]. Figure5:Cyclic Voltammogram of SPE-A2/1:8 film. Differential
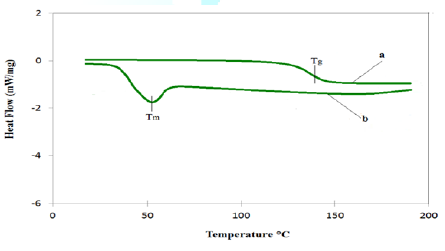
scanning calorimetry: The DSC curves of the two films,
PEO-(Li)2-XPS (curve-a), and PEO control (curve-b) are shown in Figure 6. Curve-b shows a sharp
endothermic peak observed at 54ºC which corresponds to the
crystalline melting Temperature (Tm) of the pure PEO electrolyte. By
contrast, there was no heat flow feature in curve-a until an endothermic
baseline shift occurred at 135ºC, corresponding to the glass
transition temperature (Tg) of the PEO-(Li)2-XPS. The
result indicates that this new polymer composite, PEO-(Li)2-XPS, is
an amorphous material, i.e., substantially free of crystalline phases. The
microspheres in the system aided in suppressing the crystallinity of the
polymer electrolyte [40]. The large surface area of microspheres could prevent
the local PEO chain reorganization, with the result of a high degree of
disorder. Similar to the polymer composite incorporated with inorganic
particles or fillers [41-43], we believe that the XPS beads destroyed the
crystallinity of the PEO, thus making charge transfer much easier in the
amorphous polymer network. In addition, the desired high Tg
overcomes the deficiencies of poor thermal stability of PEO, associable with
the high crystallinity and low glass transition temperature (-61ºC)
of PEO. Figure6:DSC traces of PEO-(Li)2-XPS and PEO Control. Thermogravimetric
analysis:
Figure 7 shows the TGA curves of
XPS-Li2-PEO versus PEO control-2. The PEO (curve-b) decomposed at
the temperature range 200-415°C with total weight loss of 99.66%.
Unlike the pure PEO, the XPS-Li2-PEO (curve-a) underwent a
three-step thermal degradation process. The first weight loss of 16.32% at the
temperature range about 150-200°C is attributed to chain
decomposition of polyetheramine (Jeffamine®), and the second weight
loss of 12.27% at about 300-350°C is attributed to chain
decomposition of the PEO. The final 61.52% weight loss of the XPS-Li2-PEO
composite occurred at the temperature range about 420-460°C, which
is visibly higher than the decomposition temperature of the pure PEO [43]. As
seen from the Figure 7, the major decomposition of the XPS-Li2-PEO
electrolyte does not occur until 420°C. Figure7:TGA curves of XPS-(Li)2-PEO and PEO Control. The electrolyte sample that showed the best ionic
conductivity, XPS-Li2-PEO was evaluated for surface image by
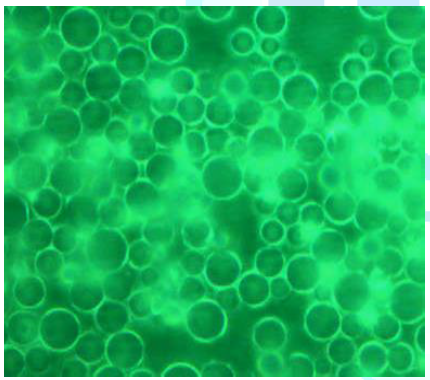
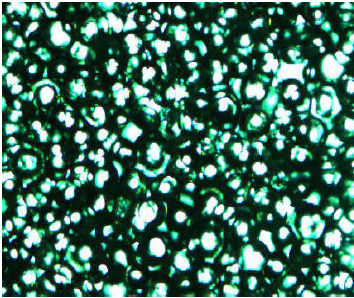
Fluorescence/DIC Microscopy. Four digital photographs were taken to describe
the morphology of this SPE material (Figure
8). In these images, the XPS beads were dispersed in a continuous phase to
form an ideal matrix for the polymer electrolyte. Figure 8a reveals that the XPS particles are spherical with a
perfectly round shape. Figure 8b
shows the light-colored material covering the XPS beads. This indicates that
the XPS microspheres were grafted by oligomeric poly(ethylene oxide) and
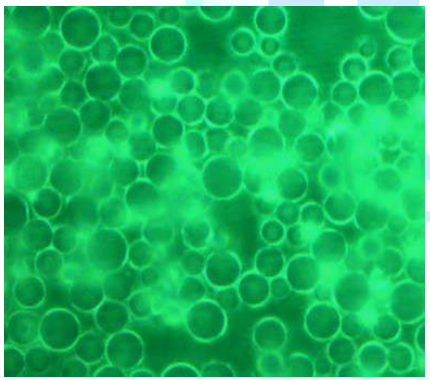
polysulfonamide derivatives. Figure 8c depicts
the phase contrast ratio of the XPS beads and the grafted PEO to be
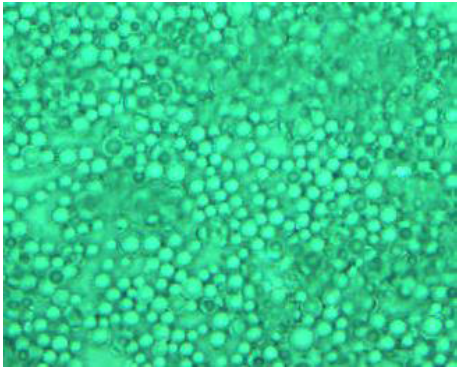
approximately 50/50 in the electrolyte matrix. Figure 8d shows the surface profile of the film, which shows a
smooth, free of defects and well-connected network. Presumably, there are
numerous pathways around on the surface of the XSP spheres. It turns out that
this morphology is favorable for enhanced three-dimensional ion transport
across the film. Figure8b:5X, reflect light source with filter.. Figure8c:20X, transmitted light source.. Figure8d:50X, reflect light source In conclusion, we have designed and synthesized two
classes of new ion-conductive SPE composites, which contain oligomeric
PEO-grafted XPS microspheres containing 1-2 lithium sulfonamide moieties. The
grafted lithium sulfonamide moieties provide a secondary source of lithium ions
that improved ionic conductivity. The SPE film containing LiTFSI in the Li:O
mole ratio of 1:8 displayed excellent ionic conductivity (1.87 x 10-4Scm-1
at 25ºC, 5.05 x 10-3S cm-1 at 70ºC),
including good electrochemical stability window up to 4.3 volts versus Li/Li+.
DSC and TGA results reveal that the new SPE is an amorphous material with a Tg
of 135ºC, and thermal decomposition temperature of 430ºC
due to the incorporation of XPS beads which destroyed crystallinity and
improved thermal stability. Morphology images of ideal surface profile were
observed, which is favorable for superior lithium ion transport. Overall, these
materials displayed good ionic conductivity, electrochemical stability and
thermal properties, resulting in improved performance when compared with the
standard LiTFSI-based PEO
electrolytes. Thus, such materials may have
potential for the development of high energy density rechargeable lithium
batteries. Braja K Mandal, Department of
Chemistry, Illinois Institute of Technology, 3101 S. Dearborn St., Chicago, IL
60616, USA, E-mail: mandal@iit.edu
Zhao W, Mei X, Yue Z and Mandal BK.
Solid polymer electrolytes derived from oligomeric poly(ethylene oxide)
chain-grafted crosslinked polystyrene microspheres (2020) Edelweiss Chem Sci J
3: 17-23. Lithium-ion batteries, Solid polymer
electrolytes, Polymer-bound lithium salts, Ionic conductivity, Cyclic
voltammetry, Optical microscopy.Solid Polymer Electrolytes Derived from Oligomeric Poly(ethylene oxide) Chain-Grafted Crosslinked Polystyrene Microspheres
Abstract
Full-Text
Introduction
Experimental

Results
and Discussion


Conclusion
References
Corresponding
author
Citation
Keywords