Research Article :
Alejandro MoureAbelenda, Kirk T Semple, Alfonso Jose Lag-Brotons, Ben MJ Herbert, George Aggidis and Farid Aiouache The present study combined two nutrient
management strategies to improve the marketability of a waste-derived
fertilizer: (a) isolation of ammoniacal nitrogen and (b) preparation of a bulk
soil amendment. The wood fly ash with low content of pollutants was added to an
agrowaste anaerobic digestate as alkaline stabilizer, which promoted the
volatilization of ammonia and adsorption processes, and as nutrient supplement.
The 39.71 ± 1.44 g blend was incubated for 60 hours at 20°C and 100 rpm in a
closed chamber (250-mL Schott Duran® bottle) with a 5.21 ± 0.10 mL
sulfuric acid trap of 10 different concentrations (0.11, 0.21, 0.32, 0.43,
0.54, 0.64, 0.75, 0.86, 0.96, and 1.07 mol/L). For analytical purposes, the
sulfuric acid, water-soluble, and water-insoluble fractions of the blend were
isolated after the incubation. The 1.07 mol/L sulfuric acid solution contained
23.69 ± 5.72 % more of ammonical nitrogen than the 0.11 mol/L solutions.
However, in all cases the amount of nitrogen in the H2SO4
compartment was lower than the one in the water-soluble and water-insoluble
fractions. Only the 15.52 ± 2.13 % of the nitrogen accounted after the
incubation was found in the H2SO4 trap. The bottleneck of
the NH3 stripping process was the rate of mass transfer at the
interface between the blended fertilizer and the headspace of the closed
chamber. The organic phosphorus was more susceptible to be adsorbed during the
alkaline treatment with non-intrusive acidification than the nitrogen and
carbon. Activation of the ash as adsorbent before mixing with the digestate
should improve the properties of the blend as slow release fertilizer, since
more nutrients would end in the water-insoluble fraction. The commercialization of waste-derived
fertilizers is constrained by the level of
contaminants [1]. It might be easier to isolate the plant nutrients contained
in the residues and sell them as conventional fertilizers [2]. The addition of
ashes to organic manures, such as anaerobic
digestates, could be proposed for: (a) improving the
properties of these organic amendments as fertilizers; (b) reducing the
greenhouse emission and phosphorus leaching associated to the management and
use of these materials; (c) manufacturing of a granular
fertilizers; and (d) increasing the pH to promote
the volatilization of ammonia and subsequent capturing the NH3 in a
sulfuric acid trap. The ammonium sulfate could be sold as liquid fertilizer
(40–60% (NH4)2SO4 solution) or as solid after
crystallization. On the other hand, the use of clean materials with low content
of pollutants, such as biomass ash and agro-industrial
digestate, enables the end-of-waste status and the
marketability of the blend as bulk soil amendment [3-9]. Mixing wood ash and agro-waste digestate to get an
approximate carbon, nitrogen, phosphorus ratio (C/N/P) of 40/2/1 could enhance
the use efficiency of these elements upon spreading the blend in the soil
[10,11]. Given the high pH of the blend ash-digestate, this material could be
used as liming agent, after the removal of the ammoniacal nitrogen (NH4+-N)
[12]. This nutrient management strategy might offer better results than the
preparation of the blend under acid conditions to promote the adsorption and to
minimize the loss of NH4+-N [13]. In fact, Miranda et al.
(2021) found that the direct addition of sulfuric acid to a 4.5% (w/v) blend of
biochar and cattle slurry mitigated less the NH3 emissions than just
applying the 0.3 mL H2SO4 (98%) to 50 g of cattle slurry
to reach a pH 5.5. The stepwise mechanism of acidification, dehydration, and
adsorption or flocculation is widely used to improve the management of
anaerobic digestates [6,14]. The present study analyzed how the H2SO4
non-invasive acidification) affects the alkaline stabilization of a blend of Wood Fly Ash (WFA)
and Post-Harvest Vegetable Waste Digestate (PVWD) in terms of: (a) NH4+-N
recovery, (b) N, C, and P availability and, (c) overall C/N/P. The severe H2SO4
non-invasive acidification was meant to decrease the amount of NH3
in the gas phase and create a greater gradient of concentration, which would be
enough to overcome the mass transfer resistance at the layer between the blend
WFA+PVWD and the headspace. The H+ that remains in the blend
WFA+PVWD due to the dissociation of WS NH4+ and
subsequent volatilization of NH3, might promote dehydration and
adsorption processes affecting the availability of nitrogen, carbon, and
phosphorus. For the preparation of the blend WFA+PVWD, the 10 mL
of ultrapure milli-Q® water was added to each gram of sample before
blending (1:10). This improved the fluency of the waste streams and generated
the Water-Soluble (WS) fraction of the blend WFA+PVWD. A detailed description
of the components of the blend WFA+PVWD is shown in the Figure 1: The 60-hour incubation of the 39.71 ± 1.44 g
fluidized blend WFA+PVWD was carried out under 100 rpm continuous shaking at 20
°C in a 250-mL (Schott Duran® bottle) closed chamber with a 5.21 ±
0.10 g H2SO4 solution to capture the NH3
released, containing an aqueous solution of H2SO4 (Figure 2). To evaluate the effect of
the non-intrusive acidification on the alkaline stabilization of the blend
WFA+PVWD, the following concentrations of H2SO4 were
tested: 0.11, 0.21, 0.32, 0.43, 0.54, 0.64, 0.75, 0.86, 0.96, and 1.07 mol/L.
The setup employed was a modification of the procedure developed by Velthof et
al. (2005), who performed 90-day incubation of manures. At every sampling
point, they refreshed the H2SO4 solution and flushed the
bottle containing the manure with N2 gas for 10 minutes, to avoid
any interference of the previous NH3
release in the next measurement. A similar procedure was
followed by Van der Stelt et al. (2007) for a 223-day incubation of dairy farm
slurry. Destructive sampling was more convenient for the present work due to
the shorter incubation. In this way, 40 experimental units (i.e. 4 repetitions
for each of the 10 H2SO4 non-intrusive acidifications)
were prepared. This methodology also offered more realistic results about the
potential of the H2SO4 non-invasive acidification to
affect the composition of the blend WFA+PVWD. The way of conducting this
experiment was based on a previous study [15] (Unpublished) and aimed that, by
the end of the treatment, all the fractions of the blend were in equilibrium,
including the H2SO4 fraction. It is important to mention
that the stoichiometric amount required to capture all the nitrogen of the
blend WFA+PVWD (Table 1) would
correspond to a 3.43-mL solution of 0.11 mol/L H2SO4. Table
1
shows the initial composition of the blend WFA+PVWD is expressed in terms of
the amount of each element in the WS extract and Water-Insoluble (WI) material.
Moreover, Table 1 includes the amount of nitrogen that would end in the H2SO4
compartment ([H2SO4] NH4+-N) at
time zero. It is important to mention that there is a share of the WS NH4+-N,
which has been volatilized and was not accounted as [H2SO4]
NH4+-N due to the mass transfer resistance. The
estimations of the organic forms of nitrogen (Norg), carbon (Corg),
and phosphorus (Porg) were based on the nature of the samples and
the empirical data of the segmented flow analysis (SEAL analytical), TOC-L
(Shimadzu), and elemental analysis (Elemental vario EL cube). The Corg
and Porg were relevant species in the blend, in the same way the
concentration of WS Norg was greater than the WS NH4+-N
and the WS NO3--N. The WS Norg was calculated as the
difference between the WS N and the sum of WS NH4+-N and
WS NO3--N. All concentrations in this manuscript were
expressed in fresh basis of the masses of WFA and PVWD used to prepare the
blend. The calculation of the concentrations of the chemical species of N, C,
and P was done using the amounts of H2SO4 solution, WS
extract, and WI material isolated after the incubation (Figure 3). A 3-µm filtration was required for the solid-liquid
separation of the WS extract and the WI material. The three fractions of the
blend were weighed with a precision balance and the calculation of the volumes
of H2SO4 solution and WS extract was done assuming a
density of 1 g/mL. The availability of an element was defined as the ratio of
the WS form to the WI form. The average recovery effectiveness of an element
was calculated as its final amount measured after the incubation divided by its
initial amount before starting the incubation. The trap effectiveness of
stripping the NH3 off the headspace was calculated as the ratio of
the [H2SO4] NH4+-N to the nitrogen
which could not be found in any of the WS and WI fractions. The One-Way Analysis
of Variance (ANOVA) was performed with Microsoft Excel
(p<0.05) to decide whether the H2SO4 non-invasive
acidification affected significantly the composition of the blend WFA+PVWD. Fractionation
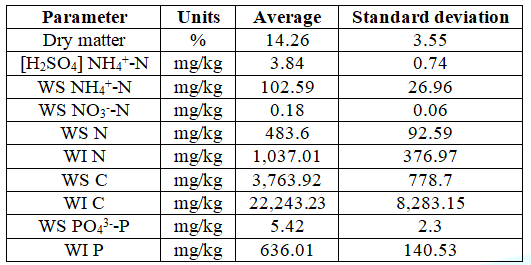
of the blend after the incubation The mass of the 5 mL H2SO4
aqueous solutions in the traps increased in agreement with the content in
sulfuric acid (Figure 3a). The 5 mL
H2SO4 solutions of 0.64 and 0.96 mol/L had lower density
than expected. The reason could be that in non-ideal solutions, the volumes are
not strictly additive. However, the data reported by Hovey & Hepler [16]
did not agree with the excess partial molar volume in that range of
concentrations for the mixtures of sulfuric acid and water. On the other hand,
it was unlikely that less volume or less concentrated H2SO4 solutions were used
instead because these trends have not been seen in any of the other results of
this study. A volume of 17.11 ± 3.45 mL of WS extract was lost
during the 60 hours incubation at 100 rpm and 20 °C and subsequent filtration
of the blends (Figure 3b). According
to the ANOVA test (p<0.05), there was not significant increase in the amount
of WS fraction recovered when using H2SO4 solutions in
the trap with greater concentration than 0.43 M. This effect would be explained
by the neutralization of the surface negative charges of the colloids of the
PVWD by adding a cationic surfactant or via intrusive acidification, which make
feasible their dehydration and flocculation. Similarly, the losses of WI
material were 0.13 ± 0.05 g and did not show dependence on the concentration of
the H2SO4
solution in the trap (Figure
3c). The explanation for the constant losses could be the procedure
followed to achieve the solid-liquid separation. Some of the WI material would
have remained stuck to the walls of the closed chamber and any weight gain due
to the hydration of the ashes would have been lost during the drying at 105°C,
before weighting the mass of the WI fraction recovered [13,14]. pH
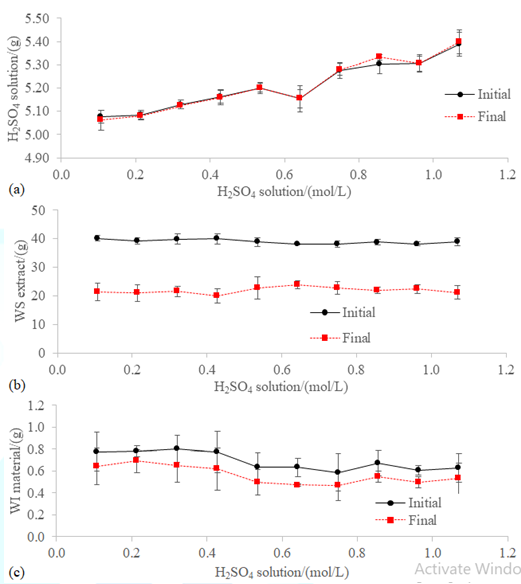
of sulfuric acid trap and the blend WFA+PVWD Since the volume of the traps were 5.21 ± 0.10 mL
(Figure 3a), the pH of the H2SO4 solutions were measured
directly (at 23.38 ± 0.36 °C) and also after eleven times dilution, to ensure a
better contact with the probe of the Mettler Toledo® Seven
CompactTM S220 pH/Ion meter. For the second set of measurements, the pH in the
undiluted traps was calculated by increasing an order of magnitude on the
concentration of the H+ species determined in the eleven times
diluted H2SO4 solutions. The first thing that needs to be
highlighted is that the pH decreased during the incubation. This is opposite to
what was expected since the absorption of NH3 should increase the pH
of the H2SO4 solutions. Understanding why the pH of the
trap decreased is important to enhance the absorption and recovery of the NH3
in the headspace. It could be possible that the pH of the trap decreased
because of the evaporation of the water and subsequent increase in the
concentration of H+ ions. However, the losses of the mass of the traps were
negligible. It should be noted that, even when the pH decreased during the
incubation, greater values than the theoretical ones were obtained. For
example, the 1.07 M H2SO4 solution should have a pH lower
than zero (i.e. -0.03) before the incubation and the value measured was 0.74 ±
0.02. After the incubation, the calculated value of pH from the measurements in
the eleven times diluted H2SO4 solutions (0.03 ± 0.06; Figure 4a) was lower than the values
measured in the undiluted traps (0.45 ± 0.05; Figure 4a). Thereby, these
calculated values of the pH could be considered more accurate than the values
of the pH obtained from the direct measurements of the H2SO4
solutions after the incubation. Therefore, the H2SO4
solutions needed to be diluted for the measurement of the variations in the
concentrations of the H+ species due to the upper detection limit of
the pH-meter. The pH of the blend WFA+PVWD was not affected by the level of
non-invasive acidification (10.40 ± 0.46; Figure
4b). Speciation of
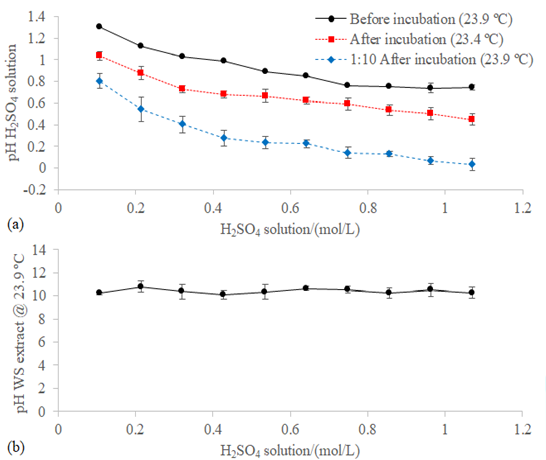
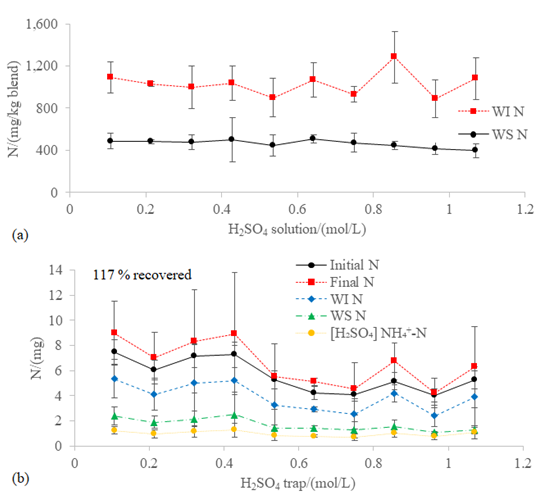
nitrogen The [H2SO4] NH4+-N
was the only nitrogen species that increased significantly (p<0.05) with
respect to the initial characterization (Table 1). The concentration of WS NH4+-N
(83.45 ± 56.99 mg/kg; Figure 5a), WS
NO3--N (0.18 ± 0.06 mg/kg; Figure 5b), WS N (463.91 ± 87.99 mg/kg; Figure 6a), and WI N (1,030.87 ± 185.20 mg/kg; Figure 6a) did not
change significantly regarding the initial characterization (102.59 ± 26.96 mg
WS NH4+-N/kg, 0.18 ± 0.06 mg WS NO3--N/kg,
483.60 ± 92.59 mg WS/kg, and 1,037.01 ± 376.97 mg WI N/kg; Table 1). High [H2SO4]
NH4+-N was expected because of the low pH of the H2SO4
solutions in the trap (0.29 ± 0.24; Figure 4a) were able to absorb the NH3
available in the headspace. The effect of the non-intrusive acidification can
be seen in Figure 5a, which shows increase (23.69 ± 5.72 %) in [H2SO4]
NH4+-N and decrease (41.08 ± 17.46 %) of WS NH4+-N
when the concentration of the H2SO4 solution in the trap
was increased from 0.11 mol/L to 1.07 mol/L. Furthermore, Figure 3b shows the
increase in the amount of WS fraction due to the presence of a H2SO4
trap with a concentration greater than 0.43 M. This significant dehydration of
the WI fraction has a p-value of 0.16. In previous work [15] (Unpublished), in
which the WFA was acidified with a 1.82 mol/L aqueous solution of hydrochloric acid
before mixing it with the PVWD, a level of 1,968.90 ± 588.36 mg WI N/kg blend
(HCl-WFA+PVWD) was reached. Since lower concentration of WI N was found in the
blend WFA+PVWD of the present study, the H2SO4
non-invasive acidification did not promote the adsorption of WS N as much as
the HCl intrusive
acidification. A calibration procedure is typically required for
measuring the concentration of NH3 in the air using H2SO4
solutions [18]. Nevertheless, in the present study, the trap effectiveness in
all the conditions evaluated was 100% (i.e. all the NH3 released to
the headspace was absorbed in the H2SO4 solutions in the
trap) and what limited the depletion of the WS NH4+-N,
was the mass transfer resistance at the film between the fluidized blend and
the gaseous phase. This constant value of trap effectiveness was calculated
without considering the amount of sulfuric acid used, which ranged from 0.05 ±
0.00 g to 0.54 ± 0.01 g of H2SO4. Otherwise, there would
be a difference of one order of magnitude between the effectiveness of the less
concentrated and the most concentrated traps. The concentration of the H2SO4
trap did not affect the volatilization of NH3 from the blend
WFA+PVWD. Similar amounts of [H2SO4] NH4+-N
were found in all the conditions evaluated (0.99 ± 0.38 mg; Figure 6b). Most of the nitrogen
recovered was in the form of WI N and only the 15.52 ± 2.13% of the nitrogen
accounted after the incubation was in the form of [H2SO4]
NH4+-N. The overall recovery effectiveness of nitrogen
was 117% (Figure 6b), as more nitrogen was accounted after the incubation than
in the initial characterization. Most of the volatilization of NH3
took place immediately after the blending and the absorption in the H2SO4
trap continued progressively during the course of the incubation, due to the
mass transfer resistance [16]. Thereby, in processes with short contact time
between the gas and the H2SO4 solution, it might be
convenient to use bubbling systems to increase surface area between the two
phases and thus the rate of transfer of NH3 [19]. As the WFA+PVWD blend had a pH of 10.40 ± 0.46
(Figure 4b), the 90 % of the WS NH4+-N was in the form of
NH3 [20]. The closed chamber continuously shaken at 100 rpm was not
enough to enable the equilibrium between the three fractions of the blend
WFA+PVWD. This setup was chosen for its low capital and operating cost but the
use of an excess of H2SO4 in the trap could not be
justified technically and economically. It might be possible to attain the
depletion of the WS NH4+-N in the blend WFA+PVWD using
advanced equipment, which allow to operate at higher temperatures under vacuum
conditions (e.g. 65 ºC and 25.1 kPa; [7]) or perform hydraulic cavitation
[21]. Another processing option would be to reduce the moisture content of the
blend, for the production of the granular fertilizer. As the surface area of
the dewatered material is greater than the fluidized blend WFA+PVWD, the
emissions of ammonia increase [5,22,23]. Speciation
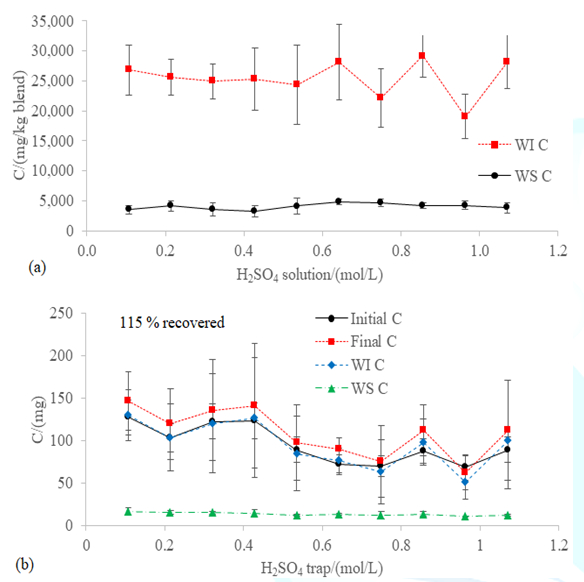
of carbon Similarly to nitrogen, most of the carbon was in the
WI form (Figure 7a). Despite the
variability of the results of WI C obtained with H2SO4 traps
with concentrations greater than 0.54 M, there was clear difference between the
concentration of the WI and the WS species of carbon. The level of WS C
(4,022.26 ± 883.39 mg/kg blend; Figure 7a) and WI C (25,350.70 ± 185.20 mg/kg
blend; Figure 7a) after the incubation was the same as in the initial
characterization (3,763.92 ± 778.70 mg WS C/kg blend and 22,243.23 ± 8,283.15
mg WI C/kg blend; Table 1). Therefore, the H2SO4 non-intrusive
acidification did not promote the adsorption of the WS Corg onto the
WFA, the release of CO2 or emission of volatile organic molecules.
It is important to mention that, Ukwuani & Tao (2016) reported the flux of
other compounds different from NH3, such as cyclohexene, towards the
H2SO4 trap. In the present study, the concentration of
the H2SO4 solution in the trap was not responsible of any
phenomena which affected the distribution of carbon between the fractions of
the blend WFA+PVWD and did not affect the recovery effectiveness of carbon
after the incubation. The alkaline pH (Figure 4b) of the blend prevented the
losses of carbon and the recovery effectiveness after the incubation was 115% (Figure 7b), which could be related to
the fact that the blend was a sink of carbon. Speciation
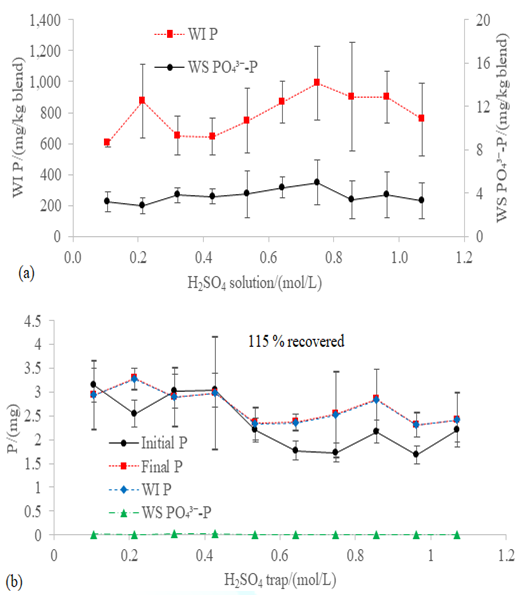
of phosphorus The concentration of WS PO43--P
(3.75 ± 1.45 mg/kg; Figure 8a) and
WI P (792.88 ± 218.92 mg/kg; Figure 8a) after the incubation was the same as
before the incubation (5.42 ± 3.84 mg WS PO43--P/kg and
636.01 ± 140.53 mg WI P /kg; Table 1). Thus, most of the phosphorus was in the
form of WI P, regardless the concentration of the H2SO4 solution
in the trap. Both the fact that there were no losses of phosphorus via gaseous
emissions in the studied conditions and the adsorption of the WS Porg
could explain the 115 % of average recovery effectiveness (Figure 8b). Any change in the amount of adsorbed WS Porg
would be accounted by the colorimetric analytical method (i.e. molybdenum blue
reaction) followed. The reason is that the sulfuric-peroxide digestion of the
WI fraction led to the formation of WS PO43--P, which was
measured with the segmented flow analysis. The availability of phosphorus went
from 0.0083 ± 0.0021 mg WS PO43--P/mg WI P at the
beginning of the incubation to 0.0048 ± 0.0015 mg WS PO43--P/mg
WI P at the end. This tiny difference in the availability could prevent losses
via leaching in an open system, for example, when applying phosphorus to land
at a rate of 26 kg/ha [10]. The fluctuations of the amount of WI N (Figure 6b),
WI C (Figure 7b), and WI P (Figure 8b) were related to the size of the system
(Figure 1) and the losses during the incubation and subsequent isolation of
this fraction (Figure 3c). The relatively small variations seen in the WS N, [H2SO4]
NH4+-N, WS C, and WS PO43--P could
be explained by the fact that less amount of nitrogen, carbon, and
orthophosphate ended up in the WS extract. Exceeding the H2SO4
non-intrusive acidification beyond the stoichiometric limit was not an
efficient way of stripping the WS NH4+-N off the blend
WFA+PVWD. The depletion of the NH3 in the headspace of the closed
chamber and the turbulence created with the 100 rpm rotary mixing did not sort
out the bottleneck of NH3 transfer from the WFA+PVWD blend to the
gas phase. Advanced processing conditions (e.g. vacuum thermal stripping and
hydraulic cavitation) or dewatering of the blend are required to increase the
rate of NH3 volatilization. Although a 10 times increase of the
concentration of the solution in the trap resulted in 23.69 ± 5.72 % more [H2SO4]
NH4+-N, this only represented an increase of 3.57 ± 2.05
% in the overall fate of nitrogen in this fraction. The C/N/P:38.74 ± 17.56/2.38
± 0.69/1 found after the incubation was the same as the intended C/N/P:40.55 ±
15.72/2.38 ± 0.80/1 (Table 1), as there was no losses during the treatment. The
greatest share of these elements was found in the WI fraction and the WS Porg
was more susceptible to be adsorbed under the studied conditions than the WS Norg
and WS Corg. This controlled-release fertilizer should minimize the
greenhouse gas emissions, eutrophication of underground waters upon land
application, and pollution swapping. Activating the WFA as adsorbent, for
example via calcination at temperatures greater than 500 ºC, could
improve the valorization of PVWD by enhancing the retention of nutrients in the
WI fraction of the blend. The authors would like to acknowledge the funding
provided by the Engineering and Physical Sciences Research Council (EPSRC,
EP/N509504/1) and the Natural Environment Research Council (NERC, NE/L014122/1)
of the United Kingdom. The EPSRC and NERC covered the cost of planning and conducting
the experiments. The interpretation of the results and preparation of the
manuscript were partly self-funded. Alejandro Moure Abelenda, Department of
Engineering, Lancaster University, Lancaster LA1 4YW, UK, Tel: +44 (0) 7933
712762, E-mail: alejandro.moure.abelenda@gmail.com
Farid Aiouache, Department of
Engineering, Lancaster University, Lancaster LA1 4YW, UK, Tel: +44 (0) 1524
593526, E-mail: f.aiouache@lancaster.ac.uk
Moure Abelenda A, Semple KT, Lag-Brotons
AJ, Herbert BMJ, Aggidis G, et al. Alkaline wood ash, turbulence, and traps
with excess of sulfuric acid do not strip completely the ammonia off an
agrowaste digestate (2021) Edelweiss Chem Sci J 4: 19-24. Waste-derived fertilizer, Alkaline
stabilization, Ammonia volatilization, Adsorption, Closed chamberAlkaline Wood Ash, Turbulence, and Traps with Excess of Sulfuric Acid Do Not Strip Completely the Ammonia off an Agro-waste Digestate
Abstract
Full-Text
Introduction
Materials
and Methods


Results
and Discussion
Conclusions
Funding
sources
References
Corresponding
authors
Citation
Keywords