Introduction
With the development of data-driven
science, the efficiency of all researchers may be improved if the rules of
data "reuse", which are different from "novelty", are
established along with the new era. Indeed, Aims and Scopes of Scientific Data published by publisher
of Nature stated “Scientific Data is a peer-reviewed,
open-access journal for descriptions of scientifically valuable datasets, and
research that advances the sharing and reuse of scientific data. We aim to
promote wider data sharing and reuse, and to credit those that share” [1].
Recently, we faced an example of his re-reporting of
the crystal structure, which was studied from another perspective. To be
honest, the cause was an omission when searching the database for previous
studies. Regarding the contents of crystal structure analysis and the latest intramolecular
interaction analysis, several previous studies were pointed out in the
paper review, and publication was refused. Curiously, it turns out that the
same crystal structure has been reported over and over again (monohydrate
5 and trihydrate 3 and other co-crystals a few times, respectively).
Discussion (from our point of view)
Metal complexes incorporating
tridentate coordinated to mersites called pincer complexes are important
for their potential applications for catalytic reactions [2]. Besides
catalysis, some copper(II) complexes having two types of tridentate ligands
were reported as a precursor of copper oxide materials derived from electrosynthesis
[3]. Herein, in order to discuss intermolecular interactions, we determined and
investigated the crystal
structure of the same complex crystallized in Pnma containing one crystalline water molecule abbreviated as
“monohydrate” (Figure 1) (our
deposition was CCDC 2115891; the first available report is [4], which was
originally prepared as a starting metal complex for further reactions by
accident. The previously reported crystal of the same complex has three
crystalline water molecules abbreviated as “trihydrate” (the first available
report is [5], in which both monohydrate and trihydrate were reported and
compared for discussion of solvent [6]).
Originally, we have investigated angles between two
characteristic mean planes (Figure 2 and Figure 3). In addition, Hirshfeld
surface analysis has been carried out for the first time [7]. For monohydrate (Figure 4), there were close contacts
with H atoms inside the surface and H atoms outside: (H-H)=16.4%, H-O=16.8%,
O-H=18.5%, C-C =11.4%, and N-C=7.3%. For trihydrate (Figure 5), the molecule A with dihedral angle is of 88.01(2)°:
H-H=16.4%, H-O=19.1%, O-H= 30.6%, C-H=8.8%, and C-O=7.6%, and another molecule
B with dihedral angle of 88.90(2)°: H-H=16.9%, H-O=18.9%, O-H=28.5%,
C-H=10.0%, and H-C=3.4% [5].
Concluding Remarks
In general, crystalline water is an important factor or phenomenon related to the phase transition of crystals. This new study of intermolecular interaction is worth reporting in that it is due to the different numbers of crystalline water, as many researchers have been reported identical crystal structures repeatedly. To discuss the intermolecular interaction, we now used Hirshfeld surface analysis, which may be relativity new or current methods for crystal chemistry. This new aspect of our study (except for crystal structure report), at least, will be expected to be applied to computational chemistry prediction of crystal structure, which is currently being researched. Therefore, it was a case where we felt that research rules that could use past data with a limited range of novelty were required.
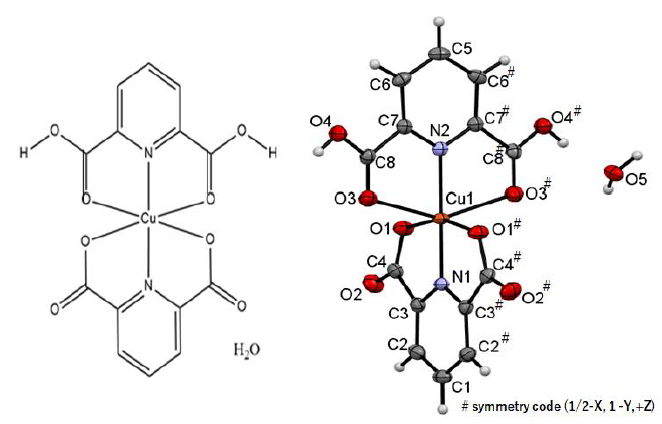
Figure1:Chemical structure of the copper(II) complex.
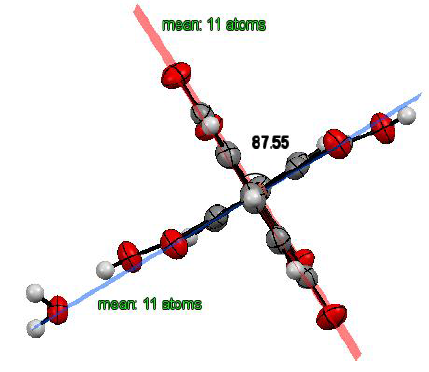
Figure2:Angle between mean-planes for monohydrate.
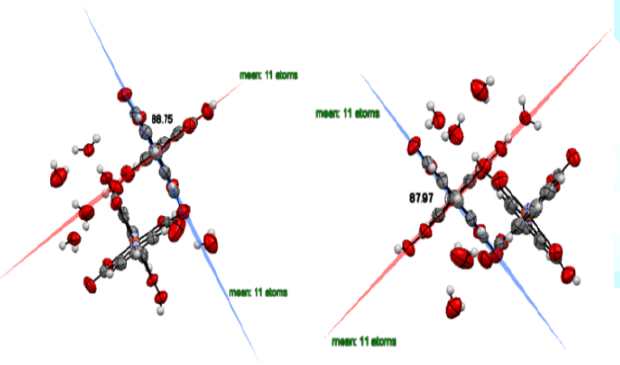
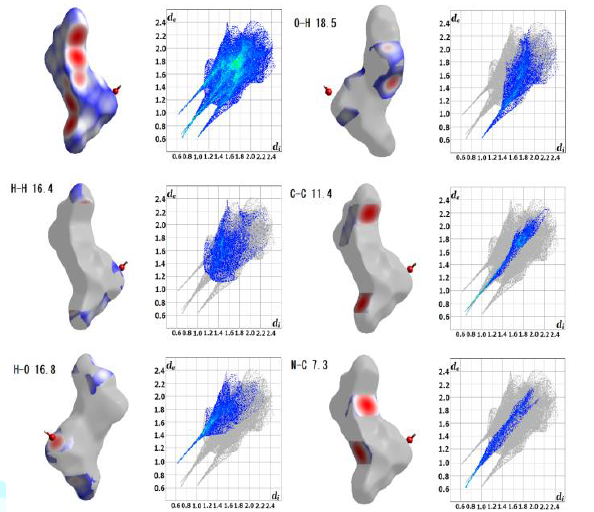
Figure4:Hirshfeld surface analysis of monohydrate.
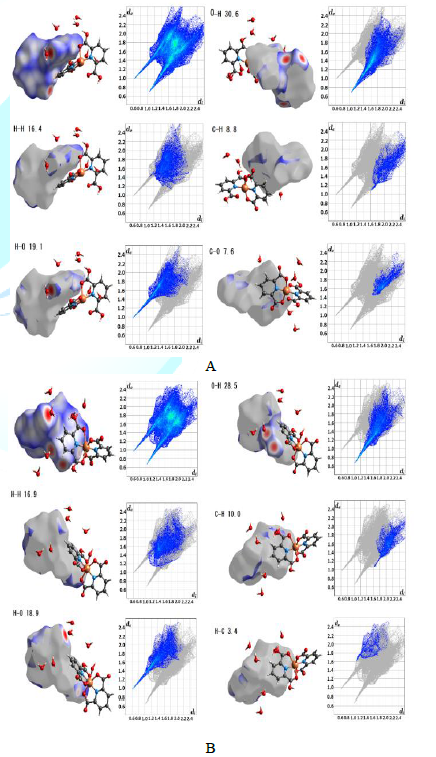
Figure5:Hirshfeld surface analysis of trihydrate for two molecules in the asymmetric unit.
Acknowledgement
This work was supported (in part) by the Nanotechnology Platform of MEXT, Grant Number JPMXP09S20NR0016. The authors thank Shohei Katao, NAIST, and Japan for X-ray crystallography. This work was supported by a Grant-in-Aid for Scientific Research (A) KAKENHI (20H00336). The authors thank Prof. Emmanuel N, Nfor’s group, Department of Chemistry, the University of Buea for providing a compound.
References
- https://www.nature.com/sdata/publish/for-authors
- Morisako
S and Yamashita M. Chemistry of pincer complexes possessing x-type boron ligand
(2019) Bull Jpn Soc Coord Chem 74: 29-45. https://doi.org/10.4019/bjscc.74.29
- Zhu Q,
Sun X, Yang D, Ma J, Kang X, et al. Carbon dioxide electroreduction to C2
products over copper-cuprous oxide derived from electrosynthesized copper
complex (2019) Nat Commun 10: 3851. https://doi.org/10.1038/s41467-019-11599-7
- Sarchet
C and Loiseleur L. Structure cristalline du [(pyridine-2,6 dicarboxylato)
(acide pyridine-2,6 dicarboxylique)]cuivre(II) hydraté (1973) Acta Cryst B29: 1345-1351.
https://doi.org/10.1107/S0567740873004486
- Sileo
EE, Blesa MA, Rigotti G, Rivero BE and Castellano EE. The crystal chemistry of
copper(II) dipicolinates (1996) Polyhedron 15: 4531-4530. https://doi.org/10.1016/0277-5387(96)00189-1
- Akitsu
T and Einaga Y.
trans-Bis(2,2-diphenylethylamine-[kappa]N)bis(5,5-diphenylhydantoinato-[kappa]N3)
copper(II) and its chloroform disolvate (2005) Acta Crystallogr 61: m183-m186.
https://doi.org/10.1107/S010827010500209X
- Spackman
MA and Sayatilaka D. Hirshfeld surface analysis (2009) Cryst Eng Comm 11: 19-32.
https://doi.org/10.1039/B818330A
Corresponding author
Takashiro Akitsu, Department of
Chemistry, Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka,
Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: akitsu2@rs.tus.ac.jp
Citation
Akitsu T, Suda S and Katsuumi N. Re-reporting
the crystal structure of copper complex from another point of view (2021)
Edelweiss Chem Sci J 4: 27-29.
Keywords
Crystal structure analysis, Hirshfeld surface
analysis, Hydrate, Intermolecular interaction, Copper complex


 PDF
PDF