Research Article :
Safaa ELMeneza, ABD ELNaser
Abdelfattah Abd ELMoean and Naglaa Abd ELmoneem Background: Neonatal Intensive Care Units (NICU) depends
heavily on biomedical devices and instruments for monitoring, diagnosis, and
treatment. The objective of this
study was to assess the impact of medical errors due to use of medical device
in NICU. Methods: This was prospective observational study to detect
medical errors due to use of medical devices in NICU in one of the governmental
hospital in Upper Egypt. It was carried out by monitoring devices related
errors over six months. The information was collected using the data entry
sheet of Egyptian Neonatal Safety Training network and designed check list. The
number and types of monitored errors as well as contributing factors were
recorded and causes were analyzed. Results: The study included 275 newborn infants, 57.45% of
them were exposed to medical device errors. The total number of recorded errors
was 215. Intravenous invasive lines devices were associated with 47.44% of
total devices errors, it was followed by respiratory equipment (26.97). The
most common cause of devices errors was due to combined failure (active and
latent) in 56.7% of total errors, followed by active failure and latent
failure, P<0.001. The frequent error in the combined devices errors was
fluid extravasation (21.3%), P<0.001, while the major cause in active
failure errors was improper use of an infusion pump (23.4%). False monitor
alarm was in 50% of latent failure errors. Majority of errors occurred during
holidays and at night shift. 61.9% of errors caused harm, adverse events were
51.6% followed by sentinel events 10.2%. Near miss was in 38.1%.
Conclusions: Invasive intravenous lines devices and
respiratory equipment were the common devices associated with errors. Adverse
events were higher than near miss and increased during holidays and night
shift. Majority of devices errors was related to combined/mixed failure.
Medical
errors are a common occurrence in the Neonatal
Intensive Care Unit (NICU). Medical error occurs due to active failure and
or latent failure. Active failure includes errors related to individuals as
doctors and nurses, while latent failure includes errors related to the system.
Faulty information management, stressful environment, inadequate training of
personnel and ineffective communication systems are some examples of latent
failures [1]. A medical device is, simply defined, any item used to diagnose,
treat, or prevent disease, injury, or any other condition that is not a drug,
biologic, or food. Medical devices range from items as simple as tongue
depressors to more complex devices, such as ventilators. Neonatal
intensive care units depend heavily on biomedical devices and instruments for
monitoring, diagnosis, and treatment [2]. Although patient safety initiatives
have focused mostly on medication errors but, medical devices also contribute
significantly to patient injuries and deaths. For this reason, the Food
and Drug Administration (FDA) put safe use of medical devices at the point of
care. However, device/equipment related errors are not a regular component of
medical education, and more researches have to carried out to understand the
root causes, implications, to avoid device errors. The impact of safety issues
related to use of devices in NICU is not entirely fully studied yet in Egyptian
NICU as far as we know after searching the midline [3]. The objective of this
study was to assess the impact of errors and adverse events due to use of
medical device in NICU using the ENSTN reporting system. The objective of this
study was to assess the impact of medical errors due to use of medical device
in NICU [4]. The
aim of this study was to determine the active and latent failures related to
devices use in NICU, and to recognize risk profile and causes of devices
errors. This
prospective observational study was carried out to monitor the medical errors
and incidents related to use medical devices in NICU. The study was performed
in one of the NICUs of governmental hospital in Assiut governorate, Upper
Egypt. The study was carried out over six months. All the newborn infants
admitted to NICU were included in the study. They had to be first time
admission to ensure non-exposure to errors in other NICUs. The study involved
275 newborn infants, 117 (42.55%) newborn infants were not subjected to medical
device error and the other 158 (57.45%) cases were exposed to medical device
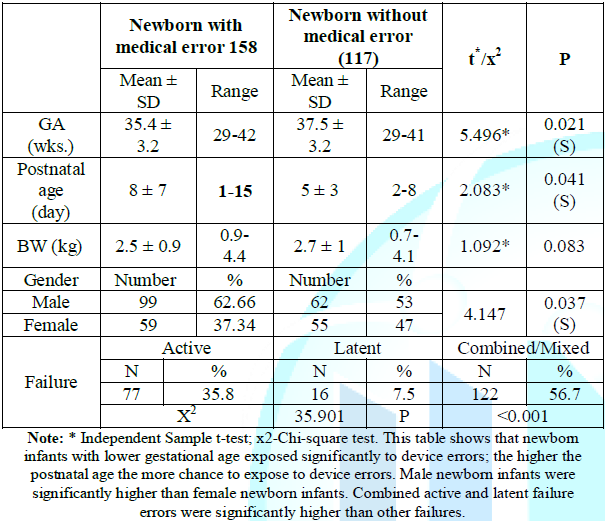
errors. The clinical characteristics are shown in Table 1. Table 1: Patients characteristics. The
non-errors group Gestational
Age (GA) ranged from 29.0 to 41.0 weeks, their Birth Weight (BW) ranged
from 0.7 to 4.1 kg and male to female ratio was 1.1:1. Their clinical diagnosis
was jaundice, Respiratory
Distress Syndrome (RDS), pneumonia, sepsis, extreme preterm infants,
diaphragmatic hernia, postoperative meningomyelocele and hydrocephalus. The
errors group GA ranged from 29.0 to 42.0 weeks, their BW ranged from 0.9 to 4.4
kg and ratio of male to female was 1.7:1. Their clinical diagnosis was
jaundice, RDS, pneumonia, sepsis and prematurity. Methods
of Data Collection This
study involved direct monitoring of patients from time of admission till
discharge, death or transfer, also review of medical records, procedures and
progress notes was performed. The patient’s history, clinical examination,
laboratory results and procedures using any medical device of involved cases
were recorded. The devices errors were collected daily through active
monitoring, using the data entry sheet of ENSTN and the designed check list for
device errors. Data collected included; type of device, nature and type of
errors resulted from device use, related risk factors; environment, human
errors or any other faults in the system that induced these errors. A
structured approach to investigate the underlying causes was used to
significantly examining the system and active causes of the resultant errors
guided by Amoore approach [4,5]. The causes of devices failure were categorized
into three types: active failure, latent failure according to Reason and mixed
failure when both system and persons were responsible for the errors. Errors
were classified according to harm into adverse, sentinel and near miss. Statistical
Analysis Data
were verified and validated then analyzed using Statistical Program for Social
Science (SPSS) version 18.0. Quantitative data were expressed as mean ±
Standard Deviation (SD). Qualitative data were expressed as frequency and
percentage. Independent-samples t-test of significance was used to compare
between two means, when data was uniformly distributed. Chi-square (X2)
test was used to compare proportions between two qualitative parameters.
Multivariate post hoc test of significance was used to compare between multiple
parameters. Probability (P-value) <0.05 was considered significant.
P-value<0.001 was considered as highly significant and P-value>0.05 was
considered insignificance. The
newborn infants with lower gestational age exposed significantly to device
errors. The higher the postnatal age the more chance to expose to device
errors. Male newborn infants were significantly higher than female newborn
infants. Combined/mixed active and latent failure errors were significantly
higher than other failures, table 1. The most common errors were related to
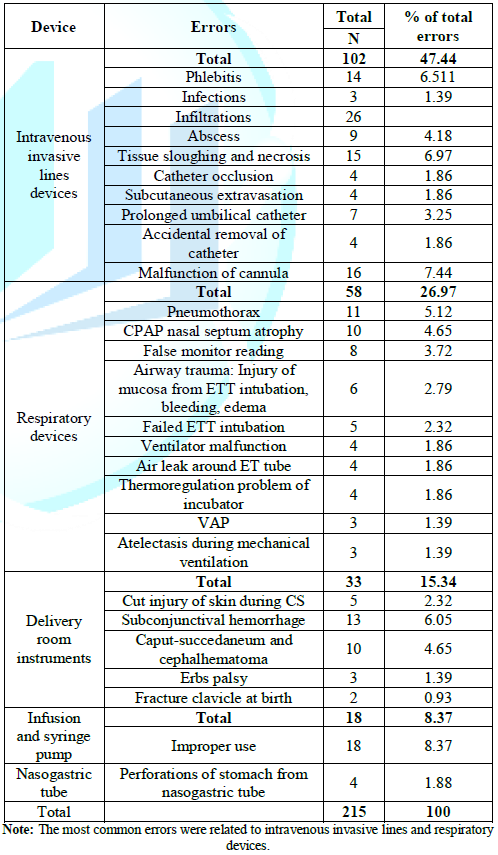
intravenous invasive lines and respiratory devices (The results are shown in Table 1 to 5). Table 2: Errors related to used devices.
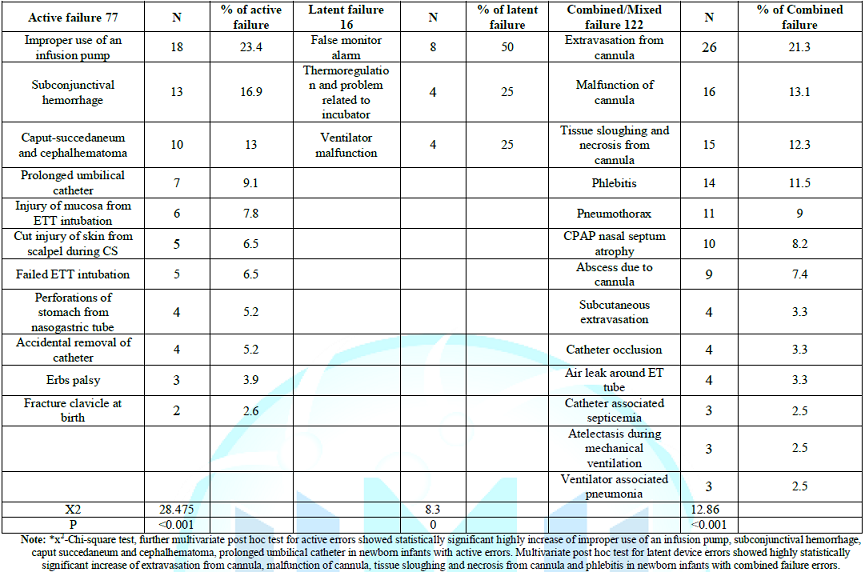
Table 3: Distribution of errors in relation to origin of failure Distribution
of errors in relation to origin of failure showed that active errors failure
had statistically significant highly increase of improper use of an infusion
pump, subconjunctival hemorrhage, caput succedaneum and cephalhematoma,
prolonged umbilical catheter in newborn infants with active errors. Latent
device errors showed highly statistically significant increase of extravasation
from cannula, malfunction of cannula, tissue sloughing and necrosis from
cannula and phlebitis in newborn infants with combined failure errors. Regarding
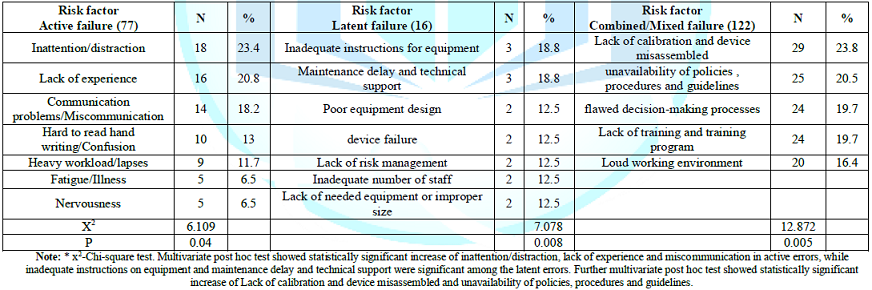
the risk factors, post hoc test showed statistically significant increase of
inattention/distraction, lack of experience and miscommunication in active
errors, while inadequate instructions on equipment and maintenance delay and technical
support were significant among the latent errors. Further multivariate post hoc test
showed statistically significant increase of Lack of calibration and device
misassembled and unavailability of policies, procedures and guidelines, Table 4. Devices errors significantly
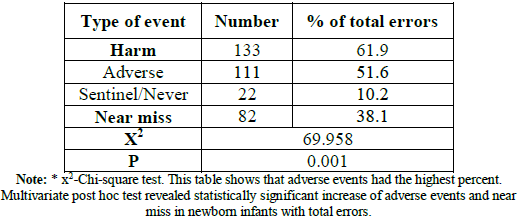
increased during holidays and during night shift, table 5. Errors with harm
(adverse and sentinel events) resulted in 61.9% of total errors, while errors
without harm (near miss) occurred in 38.1%. The
errors that cause injuries to patient resulting from the management by the
medical staff is defined as adverse event. Active or person approach focuses on
the errors of individuals, blaming them for forgetfulness, inattention, or
moral weakness. The system or latent approach concentrates on the conditions
under which individuals work and tries to build defenses to avert errors or
mitigate their effects [1]. This study was prospective observational study to
identify medical
device errors. Confidentiality, non-punitive, anonymous, and voluntary
reporting system was used. Table 5: Classification of devices errors in relation to type of event Voluntary,
nonpunitive systems are thought to yield the best estimate of iatrogenic
events, the error rate was much higher in studies using voluntary reporting
than in those based on mandatory reporting [4,6]. The current study included
275 newborn infants, 57.45% of them were subjected to medical device errors.
The most common devices associated with errors was related to intravenous
invasive lines devices (47.44%) followed by respiratory devices (26.97%) then
delivery room instruments (15.34%), infusion and syringe pump (8.37%) and
nasogastric tube (1.88%). These findings were comparative to other studies;
Bergon-Sendin, et al., found that the rate of appropriate use of the monitors
and respiratory support equipment was 33.68%, which mean that 66.32% was
inappropriate use; Snijders, et al., reported that 41% of incidents were due to
mechanical
ventilation and intravascular catheters. In
report from ENSTN equipment errors were 31.34% from the total reported errors. The
higher percentage in the current study may be due to involvement of errors from
all medical devices including simple devices as peripheral catheters, infusion
pump, endotracheal tube as well as complicated equipment as monitors and
ventilators [7-9]. The variations in incidence between different studies were
due to inconsistency of definitions, type of involved devices as well as
discrepancy in type of the studies and the nature of reported errors. In this
study all incidents were reported weather lead to harm as adverse event or no harm
as near miss. This study showed that significant increase in errors related to
combined active and latent failure than errors of latent or active failure
P<0.001. This finding denotes that jointly system and personal approach are
important in preventing devices related errors. Active
failure includes errors related to individuals as doctors and nurses, while
latent failure includes errors related to the system. Faulty information
management, stressful environment, inadequate training of personnel and ineffective
communication systems are some examples of latent failures [1]. Combined/Mixed
failure errors resulted in several harm included peripheral catheter related
lesions as extravasation from cannula in 21.3%, multivariate post hoc analysis
showed also that malfunction of cannula (13.1%), tissue sloughing and necrosis
(12.3%) and phlebitis of grade 1to III (11.5%) were highly significant too,
P<0.001. Abscess was detected in 7.4%, umbilical catheter occlusion in 3.3%,
and umbilical catheter associated septicemia occurred in 2.5% of the reported
errors. Other studies reported infiltration/extravasation in 45.6% of
peripheral venous catheters inserted into newborns and incidence of contracting
extravasation harm producing skin necrosis in 38/1000 babies admitted to
tertiary NICUs. Reichembach,
et al., found the incidence of complications related to use of intravenous
peripheral catheters in 63.15% of neonates, being infiltration/extravasation
(69.89%), phlebitis (17.84%) and obstruction (12.27%) [10-12]. The significant
risk factors of combined/mixed errors were lack of calibration and device
misassembled (23.8%) and unavailability of policies, procedures and guidelines
(20.5%), it was followed by flawed decision-making processes (19.7%), Lack of
training and training program (19.7%) and loud working environment (16.4%), P=0.005.
Several contributing factors include; inattention, improper cannulation, poor
quality of used catheters, use of fluids containing calcium
and hypertonic fluids, absence of flushing before and after administration
of medications, unavailability of policy, procedures and guidelines, prolonged
use of the cannula in same vein, use of incorrect cannula gauge, and trauma to
vein during insertion. These risk factors were comparable to others. Thus, even
simple device as peripheral intravenous cannula/catheter can predispose to
several medical errors and harm from simple extravasation to tissue necrosis [12,13].
Pneumothorax was detected in 5.12% of total errors in ventilated newborn infants;
the root causes were related to improper intubation, high
peak inspiratory pressure, high volume, and inadequate monitoring and
follows up. Air
leak around endotracheal tube happened in 1.86% of errors and related to the
incorrect position or improper size of tube. Atelectasis during mechanical
ventilation also occurred in 1.39%, and was due to mucous plug, poor
suctioning, and right
main stem intubation. Ventilator associated pneumonia was 1.39% of errors
with combined failure which related to unavailability of antiseptic guidelines,
prolonged mechanical ventilation, frequent re-intubation, prematurity, and low
birth weight. Nasal septum atrophy due to CPAP was 4.65% of combined/mixed
failure errors, and was related to prematurity, improper prong placement/size
and long duration of use. Schuman et al found that incidence of
pharyngoesophageal injury was 0.10% and increased with decreasing gestational
age and weight being 0.38% at less than 27 weeks’ gestation [14]. Several
studies depicted the complications related with mechanical ventilation. It was
recognized that prolonged mechanical ventilation was related to BPD,
pneumothorax, errors related to endotracheal intubations as malpositioning,
trauma to the vocal cords, larynx, and esophagus, subglottic stenosis and
palatal grove, and ventilator-associated pneumonias [6,15]. This study showed
the magnitude of the combined failure errors that occurred due to both active
and latent failure. Latent conditions additionally dispose to the happening of
numerous active failures including slips, lapses, mistakes and violations that
ensue in patient harm. Latent disorders reduce the barriers of defense against
error [16]. Majority
of errors caused by active failure were improper use of an infusion pump in
23.4%, followed by sub conjunctival hemorrhage in 16.9%, caput
succedaneum and cephalhematoma in 13.0% and prolonged use of umbilical
catheter in 13.0% of errors due to active failure, P˂0.001. Errors of use of
infusion pump were related to incorrect settings, calculation error in rate.
Theses might be due to inattention/distraction, poor communication between
staff, lack of expertise, heavy workload, fatigue or more often poor
documentation of administration and unavailability of guidelines. It was also
reported that infusion pump programming errors is most common cause as well as
improper use of an infusion pump [17]. Other
active failure errors were prolonged use of umbilical catheter>14 days (3.25%),
mucosal injury due to ETT (2.79%), failed ETT (2.32%), cut injury of skin
during cesarean section (2.32%), perforation of stomach from nasogastric tube
(1.86%), accidental removal of umbilical catheter occurred (1.86%), Erbs palsy
(1.39%), and fracture of clavicle at birth (0.93%). Birth trauma was reported
by other studies and reported as 3.1% in labor room admissions [18,19]. The related
causes to active failure were inattention/distraction, lack of experience,
miscommunication, confusion, heavy workload/lapses, fatigue/illness, and
nervousness. The
root causes included unavailability local guidelines and training that were
augmented by lack of experience of the newly hired residents and nurses and
inadequate supervision by senior staff. Lack of training and experience was
found in 10.5% of cases. Also there was misinterpretation of medical orders.
Sometimes patient related factors as large for gestational age and prematurity.
These factors may amplify the incorrect thought processes, reasoning or
analysis among stressed and extreme cognitive loaded staff and lead to lack of
concentration slips, lapses or fixation that lead to mistakes [19]. Regarding
the latent errors, this study showed that false interpretation of alarm and
monitor reading (50%) was significantly higher than other causes of latent
failure errors. The alarm problems were studied in other NICU and reported to
be very significant. In our study, the other causes of latent failure included
ventilator malfunction which was recorded in 25% of errors related to latent
failure. These results agreed with others. Parihar reported equipment
failures or inadequacy in 14.8% of the errors. Thermoregulation and problem
related to incubator was detected in 25% of errors, other study focused on
errors related to incubator, showed that 40% of incubators showed output
temperature higher or lower than specified limits for each measurement point
[8,9,20,21]. Causes
related to latent failure included inadequate instructions for equipment that
lead to inappropriately use, delay maintenance, technical support, device
failure; incorrect connection, occlusion, incorrect usage and material damage,
lack of needed equipment or improper size, poor equipment design. Root causes
were related to insufficient or incorrect training related to procedures,
machine and teamwork that lead to lack of skills to operate medical devices,
also lack of proper suppliers, purchasing poor quality catheters, and
inadequate policies, procedures and guidelines as well as constant
communication methods. Lacking of calibration and organization maintenance
affect precision of monitor displays and alarm failure triggered staff misread
of records or overlook it with subsequent unsuitable clinical judgments [3]. The
gestational age was significantly lower among newborn infants subjected to
device errors than non-device errors newborn infants. These results were in
coincidence with the data of others. The increased liability to device errors
in preterm infants may be due to their need for assisted ventilation and use of
medication and intravenous fluid than other newborn infants. The rate of
incident per neonate was 1.27. Our results showed that the device errors
occurred more in males than females as percent of male to female was 1.7:1.
This reflect the increase of admission of male newborn infants than female
newborn infants in the studied NICU, this is similar to Hoffmeister, et al.,
who reported 52.9% male gender in their study and frequency of 1.6 incidents
per newborn [22-24]. There
was significant increase in devices errors during holidays (63.3%) than
workdays (36.7%). Also significant higher errors occurred at night shift
(50.7%) than morning (19.5%). Work overload time shift potentiate to more
medication errors [23]. Concerning to type of event, our study showed that
majority of errors were adverse events, it resembled 51.6% of events. The
literature showed variable data due to different definition, methods of
evaluation of errors, gestational age and methodology. One study reported
adverse events in 57% at gestational ages of 24 to 27 weeks compared with term gestation
(3%). Adverse events occurred in 39% intubations with non-severe and severe
events in 35% and 8.8% intubations, respectively. The overall adverse event
reported by ENSTN was 43.2% while close call or near miss was 50.16%, 5.9% was
sentinel events and 0.222% considered as unsafe act [6,9,25]. Conclusion This
study was an attempt to brief the healthcare personnel to the importance of
medical device errors. Invasive lines devices and respiratory equipment were
the common devices associated with errors. The errors were either due to user
or equipment malfunctions. Identifying technical failure/equipment malfunctions
from user errors is an important to inhibit adverse events. Adverse events were
higher than near miss and increased during holidays and night shift. Majority
of devices errors was related to combined/mixed failure. It means that system
errors predispose to active errors and from this perspective, improvement of
safety issues related to devices need system and personal approach. Ethical
Considerations The
study was approved by Faculty of Medicine of Girls, Al-Azhar University
council. According to the ENSTN reporting system, confidentiality was ensured.
Parents’ consent was obtained after explaining the nature of the study.
We
acknowledge the parents of the participated infants for their support and
acceptance to participate in the study. Also the nurse staff who helped the
researcher during collecting the data.
Medical device errors, Neonatal safety, Egyptian
Neonatal Safety Training network (ENSTN), Active failure, Latent failure.Study of Medical Errors Triggered by Medical Devices in Neonatal Intensive Care Unit
Abstract
Full-Text
Introduction
Objectives
Methods
Results
Discussion
Acknowledgements
References
Corresponding
author
Safaa ELMeneza, Pediatrics department,
Faculty of Medicine for Girls, AL-Azhar University, Egypt, Email:
safaa5@hotmail.comCitation
ELMeneza S, Abd ELMoean AFAN and Abd ELmoneem N. Study of medical errors triggered by
medical devices in neonatal intensive care unit (2020) Edelweiss Pediatrics J 1: 7-12. Keywords