Introduction
U-74389G is a novel Lazaroid (L) antioxidant factor implicating just only 261 published studies. The Ischemia Reperfusion (IR) type of experiments is noted in 19.15% of these studies. A tissue protective feature of U-74389G is obvious in these IR studies. The U-74389G chemically known as 21-[4-(2,6-di-1-pyrrolidinyl-4-pyrimidinyl)-1-piperazinyl]-pregna-1,4,9(11)-triene-3,20-dione maleate salt is an antioxidant complex, which prevents the lipid peroxidation either iron-dependent, or arachidonic acid-induced one.
Animal kidney, liver, brain microvascular endothelial cells monolayers and heart models are protected by U-74389G after IR injury. U-74389G also attenuates the leukocytes; down-regulates the proinflammatory gene; treats the endotoxin shock; produces cytokine; enhances the mononuclear immunity; protects the endothelium and presents antishock property. 4 histologic variables in a Uterine Ischemia Reperfusion (UIR) experiment were tested for this purpose. The two variables were those of Endometrial Edema (EE) and Uterus Inflammation (UI) which had a non-significant recessing potency at the without lesions grade 0.2636364 ± 0.14594051 (p-values=0.0698) since they were co-evaluated together [1]. The other variables were those of Endometrial Karyorrhexis (EK) and Uterus Congestion (UC) which had a non-significant recessing potency at the without lesions grade 0.1253529 ± 0.08529668 (p-values=0.1373) since they were co-evaluated together [2]. The present experimental work tried to co-evaluate these EE, EK, UC and UI variables together and to end up to their outcome totally, from the same rat induced UIR protocol. The paradox of this study is that, taking into account two recessing studies, it yielded an accentuating one concerning the histologic variables.
Materials and methods
Animal preparation
The study received 2 ethics committee approvals under the 3693/12-11- 2010 & 14/10-1-2012 numbers fully following the tenants of the Declaration of Helsinki. The granting company, the experiment location and the Pathology Department are mentioned in preliminary references [1,2]. The human animal care of Albino female Wistar rats, the 7 days pre-experimental ad libitum diet, the non-stop intra-experimental anesthesiologic techniques, the acidometry, the electrocardiograms, the oxygen supply and the post-experimental euthanasia are also described in preliminary references. Rats were 16-18 weeks old. They were randomly assigned to four (4) groups consisted in N=10. The stage of 45 min ischemia was common for all 4 groups. Afterwards, reperfusion of 60 min was followed in group A; reperfusion of 120 min in group B; immediate L intravenous (IV) administration and reperfusion of 60 min in group C; immediate L IV administration and reperfusion of 120 min in group D. The dose height assessment was described at preliminary studies as 10 mg/Kg body mass.
Ischemia was caused by laparotomic clamping the inferior aorta over renal arteries with forceps for 45 min. The clamp removal was restoring the inferior aorta patency and reperfusion. After the blood flow interruption, the protocols of UIR were applied, as described above for each experimental group. L was administered at the time of reperfusion; through inferior vena cava catheter.
The EE, EK, UC and UI scores were determined at 60th min of reperfusion (for A and C groups) and at 120th min of reperfusion (for B and D groups). Relation was raised between animals mass with neither EE scores (p-value=0.7779) nor with UI ones (p-values=0.0576), nor with EK scores (p-value=0.8683) but only with UC ones (p-values=0.0047); hence the predicted UC scores were used. The pathologic score grading was maintained the same as in preliminary studies: (0-0.499) without lesions, (0.5-1.499) for mild lesions, (1.5-2.499) for moderate lesions and (2.5-3) for serious lesions damage. The paradox of this study is that, taking into account two recessing studies, it yielded an accentuating one concerning the histologic variables.
Model of ischemia-reperfusion injury
Control groups: The 20 control rats were the same for preliminaries and this study (Table 1).
Group A: Reperfusion which lasted 60 min concerned 10 controls rats of combined EE, UI, EK and UC Uterine Score (cUS) as the mean of EE & UI scores and the EK & predicted UC ones.
Group B: Reperfusion which lasted 120 min concerned 10 controls rats of combined EE, UI, EK and UC Uterine Score (cUS) as the mean of EE & UI scores and the EK & predicted UC ones.
L group: The 20 Epo rats were the same for preliminaries and this study.
Group C: Reperfusion which lasted 60 min concerned 10 L rats of combined EE, UI, EK and UC Uterine Score (cUS) as the mean of EE&UI scores and the EK & predicted UC ones.
Group D: Reperfusion which lasted 120 min concerned 10 L rats of combined EE, UI, EK and UC Uterine Score (cUS) as the mean of EE & UI scores and the EK & predicted UC ones.
Statistical Analysis
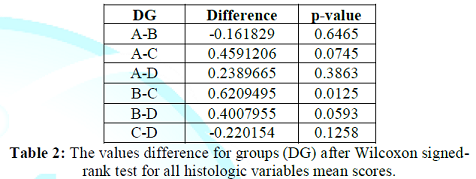
Every cUS groups score was compared with each other from 3 remained groups applying Wilcoxon signed-rank test (Table 2). Then, the Generalized Linear Models (GLM) were applied with dependent variable the cUS scores, and independent variables the L administration or no, the reperfusion time and their interaction.
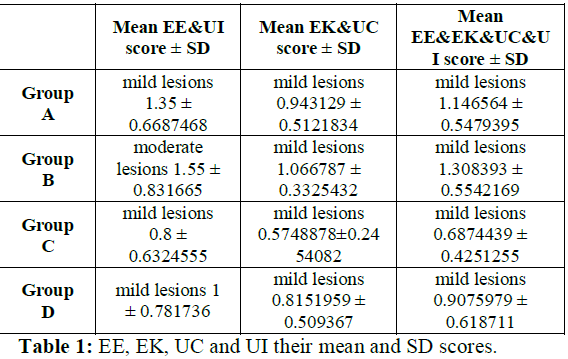
Table 1: EE, EK, UC and UI their mean and SD scores.
Results
L administration non-significantly recessed the 4 histologic variables within the without lesions alterations score 0.24361355 [-0.57263045- -0.227816] (p-value=0.3830), after co-calculation by both Wilcoxon signed-rank test and glm methods.
Contrary, reperfusion time non-significantly accentuated the 4 histologic variables within the without lesions alterations score 0.19002235 [-0.1554684 - +0.5355131] (p-value=0.2707), after co-calculation by the same methods. Totally, L administration and reperfusion time together non-significantly accentuated the 4 histologic variables within the without lesions alterations score 0.0758471 [-0.1464624 - +0.2981566] (p-value=0.4940) (Tables 3 and 4).
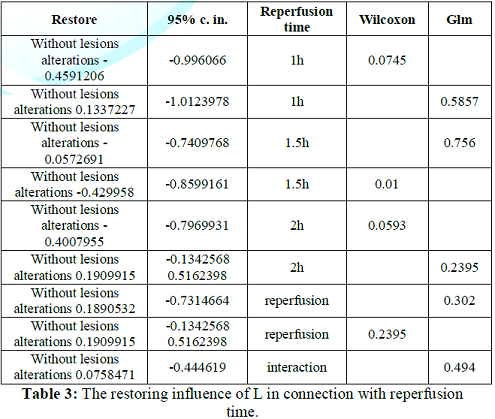
Table 3: The restoring influence of L in connection with reperfusion time.
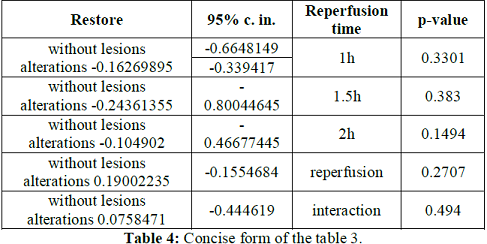
Table 4: Concise form of the table 3.
Discussion
Tsuji M, et al., consider UIR as one of the major causes of intrauterine/fetal growth restriction, preterm birth, and low birth weight [3]. The offspring of their Mild Intrauterine Hypo-perfusion (MIUH) model clearly demonstrates long-lasting alterations in neurological, neuroanatomical and behavioral test results. Ugurlu T, et al., showed that antioxidant acetyl L-carnitine that added to the organ preservation solution (Post-Partum Hemorrhage) HTK, has prevented the formation of free radicals and mitochondrial damage, thus protects the uterus that was stored in short and long cold storage periods in female rats [4].
UIR is a complex pathophysiological process involved in hypoxia and/or reoxygenation (UHR), ionic imbalance-induced edema and acidosis, oxidative stress, mitochondrial uncoupling, coagulation and endothelium activation. VEGFR-2 plays an important role in angiogenesis, chemotaxis, proliferation and migration of endothelial cells. Sholapurkar SL, et al., proposed an ischemia and mal-apposition hypothesis for Cesarean Scar (CS) niche, stating that the surgical technique of uterine incision closure is the most important determinant of CS defect formation [5]. Single-layer technique may be best reserved for thin myometrial edges especially during repeat cesareans. Alotaibi M suggested that a mechanism of uterine tolerance (preconditioning) is confined to uterine tissues very close to labour and it is a protective phenomenon to improve the uterine activity despite the long-lasting paradoxical metabolic challenges that occur during the repeated strong labour contractions in rat uterine term-pregnant tissues [6]. Ren Z, et al., showed that correcting soluble fms-like tyrosine kinase-1 (sFlt-1)/Placental Growth Factor (PlGF) imbalance by infusing PlGF reverses the decreases in vascular and uteroplacental Matrix Metallo Proteinase (MMP)-2 and MMP-9 and the increases in MMP-1, MMP-7, and collagen types I and IV induced by placental ischemia and antiangiogenic sFlt-1 in hypertension of pregnancy [7].
Angiogenic factors and MMP modulators could rectify changes in vascular and uteroplacental MMPs and collagen content and ameliorate hypertension and intrauterine growth restriction in preeclampsia. Clayton AM, et al., claims that placental ischemia, induced by Reducing Uterine Perfusion Pressure (RUPP), leads to cerebral edema and increased blood-brain barrier permeability and thus increased mortality risk from Alzheimers disease, stroke, and cerebrovascular complications in women with a history of preeclampsia in pregnancy [8]. Astrocyte number was increased in both regions but area covered by astrocytes increased only in posterior cortex following RUPP. Posterior cortical occludin was decreased. These results suggest that 2 months postpartum, neuroinflammation, along with decreased occludin expression, may partly explain posterior cortical edema in rats with history of placental ischemia. Simoni M, et al., considered insufficient stem cell recruitment to adequately repair the uterus resulting in conditions such as Asherman syndrome, endometriosis and other endometrial receptivity defects [9].
In contrast, excessive recruitment of stem cells underlies endometriosis. Further, the normal endometrium is a rich source of multipotent stem cells that can be used for numerous applications in regenerative medicine beyond reproduction. Stem-cell mobilization inhibiting may also be helpful in endometriosis therapy. Almohanna AM, et al., suggested what may be general mechanisms of conditioning occurring in all smooth muscles and tabulated tissue-specific mechanistic findings [10]. Kisu I, et al., achieved the first delivery after autologous uterus transplantation (UTx) in primates and the first periodic recovery of menstruation after allogeneic UTx in nonhuman primate models [11]. In addition, more validation in nonhuman primate models is needed for resolution of medical issues and further development of UTx in humans, despite clinical application of UTx in several countries. Vaka VR, et al., found mitochondrial ROS significantly elevated in endothelial cells incubated with RUPP serum compared with normal ones [12].
Impaired mitochondrial function and vascular, placental, and renal mitochondrial ROS play an important role in hypertension and reduced fetal weight in response to placental ischemia during pregnancy in female pregnant Sprague Dawley rats. Koizumi N, et al., submitted a 41-year-old woman [13]. In an elective needlescopic operation using 2- and 3-mm instruments after bowel decompression out of right broad ligament hernia. The defect in the right broad ligament was closed with sutures and she was discharged 2 days after the operation. In the treatment of broad ligament hernia without bowel ischemia, neither an abdominal incision nor any energy devices are required. Needle scopic operation seems to be a promising approach among minimally invasive operations. Kopko J, et al., found intra-operatively acute appendicitis and levorotation of the pregnant uterus at 19th week of gestation by about 100 degrees [14].
As the signs of ischemia were absent, the uterus was returned into its normal position. The patient underwent cesarean section at 36 weeks of pregnancy due to early leakage of amniotic fluid and failure to progress during first stage of labor. Padma AM, et al., distinguished reperfusion injury-related differences associated with organ preservation; that may lead to improved human uterus transplantation protocols curing women with uterine factor infertility. A much faster and severe reperfusion damage of all uterine layers during the reperfusion experiment was got evident following 48 hours of cold ischemia [15]. This was indicated by major accumulation of extracellular fluid, presence of apoptotic-labeled glandular epithelial layer and vascular endothelium. A significant accumulation of lactate was measured in the perfusate with a subsequent decrease in pH in a novel ex vivo sheep uterus model. Chen C, et al., assessed the safety and efficacy of Transcatheter Arterial Embolization (TAE) of the Inferior Mesenteric Artery (IMA) for the management of Post-Partum Hemorrhage (PPH) [16].
Bleeding from the IMA should be suspected when there is persistent vaginal bleeding after sufficient embolization of bleeders from the bilateral iliac arteries. Saat N, et al., indicated that melatonin improved fertility and reduced uterine torsion related tissue damage and that its application during torsion was more effective than application following removal of torsion in pregnant rats [17]. Tardieu A, et al., demonstrated hypoxia-associated degradation of the organ by the significantly higher lactate levels, accompanied by cell lysis and significantly higher levels of creatine kinase activity (p<0.05) [18]. The metabolic results indicate a significant degradation of the uterus during 24 h of Cold Ischemia (CI) before transplantation in explanted ewes uteri. Tardieu A, et al., calculated the mean CI time in studies of births from uteri obtained from live donors between 2 h 47 min and 6 h 20 min from a deceased donor; with only one birth in this case in women [19].
Muscle contractions have also been demonstrated in myometrial samples from women, after six or more hours of CI. The uterus seems to be able to tolerate a prolonged period of CI, of at least six hours; for the development of UTx, particularly for procedures using grafts from deceased donors. Along with other authors, we believe that the initial step resulting in uterine damage is an acute increase in lipid peroxidation following ischemia, which damages endometrium and other myometrial cells that ultimately induce their own apoptosis. In theory, high levels of lipid peroxidation are decreased over the course of reperfusion. During this time, either the cells may be destroyed via apoptosis or they remain alive but show signs of damage such as the 4
Mentioned histologic features if oxidative damage is not intense enough. We hypothesize that lipid peroxidation may decrease following an oxidative insult as the cell overcompensates its antioxidant efforts to counteract such an insult. Despite this rigorous antioxidant effort by the cells, the damage may be too great and continue its course, and progressive destruction leads to the greatest loss observed at the endpoints of reperfusion. A numeric evaluation of the L efficacies was provided by a meta-analysis of 35 seric variables of complete blood count and blood chemistry tests versus reperfusion time coming from the same experimental setting [20] (Table 5).

Conclusion
L administration non-significantly accentuated the 4 histologic variables within the without lesions alterations score 0.0758471 [-0.1464624 - +0.2981566] (p-value=0.4940) disputing for beneficial usage in obstetric situations such as intrauterine/fetal growth restriction, preterm birth, hypertension, low/reduced fetal weight due to placental ischemia, uterine activity during the repeated strong labour contractions, preeclampsia, after bowel decompression out of right broad ligament hernia or correction of a rotated pregnant uterus, uterine torsion, post-partum hemorrhage and cesarean scar niche formation.
Many gynecologic situations also could be benefited such as endometriosis therapy, Asherman syndrome, Alzheimers disease, stroke, posterior cortical edema and cerebrovascular complications in women with a history of preeclampsia, angiogenesis, chemotaxis, proliferation and migration of uterine endothelial cells in regenerative medicine beyond reproduction and development of autologous or allogeneic stored uterus transplantation protocols curing women with uterine factor infertility.
References
- Τsompos C, Panoulis C, Τοutouzas K, Triantafyllou A, Ζografos G, et al. The co-evaluation of endometrial edema and uterus inflammation after the u-74389g effect on uterine ischemia reperfusion injury (2017) J Contracept Stud 2:12. https://doi.org/10.21767/2471-9749.100039
- C. Tsompos, C. Panoulis, K. Toutouzas, A. Triantafyllou, GC. Zografos, et al. The co-evaluation of endometrial karyorrhexis and uterus congestion after the u-74389g effect on uterine ischemia reperfusion injury (2018) EC Proteomics and Bioinformatics 2: 65-68. https://doi.org/10.28982/josam.400277
- Tsuji M, Coq JO, Ogawa Y, Yamamoto Y and Ohshima M. A rat model of mild intrauterine hypoperfusion with microcoil stenosis (2018) J Vis Exp 131: e56723. https://doi.org/10.3791/56723
- Ugurlu T, Ozogul C, Saribas GS, Gurgen SG, Akyol SN, et al. The effect of antioxidants on angiogenesis in uterine transplantation (2018) J Obstet Gynaecol 38: 382-387. https://doi.org/10.1080/01443615.2017.1316250
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate (2018) J Clin Med Res 10:166-173. https://doi.org/10.14740/jocmr3271w
- Alotaibi M. Brief hypoxic cycles improve uterine contractile function after prolonged hypoxia in term-pregnant but not in nonpregnant rats in vitro (2018) Theriogenology 113:73-77. https://doi.org/10.1016/j.theriogenology.2018.02.011
- Ren Z, Cui N, Zhu MA and Khalil RA. Placental growth factor reverses decreased vascular and uteroplacental MMP-2 and MP-9 and increased MMP-1 and MMP-7 and collagen types I and IV in hypertensive pregnancy (2018) Am J Physiol Heart Circ Physiol 315:H33-H47. https://doi.org/10.1152/ajpheart.00045.2018
- Clayton AM, Shao Q, Paauw ND, Giambrone AB, Granger JP, et al. Postpartum increases in cerebral edema and inflammation in response to placental ischemia during pregnancy (2018) Brain Behav Immun 70: 376-389. https://doi.org/10.1016/j.bbi.2018.03.028
- Simoni M and Taylor HS. Therapeutic strategies involving uterine stem cells in reproductive medicine (2018) Curr Opin Obstet Gynecol 30:209-216.
- Almohanna AM and Wray S. Hypoxic conditioning in blood vessels and smooth muscle tissues: effects on function, mechanisms, and unknowns (2018) Am J Physiol Heart Circ Physiol 315: H756-H770. https://doi.org/10.1152/ajpheart.00725.2017
- Kisu I, Banno K, Matoba Y, Adachi M and Aoki D. Basic research on uterus transplantation in nonhuman primates in Japan (2018) J Obstet Gynaecol Res 44:1871-1881. https://doi.org/10.1111/jog.13724
- Vaka VR, McMaster KM, Cunningham MW Jr, Ibrahim T, Hazlewood R, et al. Role of mitochondrial dysfunction and reactive oxygen species in mediating hypertension in the reduced uterine perfusion pressure rat model of preeclampsia (2018) Hypertension 72: 703-711. https://doi.org/10.1161/hypertensionaha.118.11290
- Koizumi N, Ariyoshi Y, Fujiki H and Sakakura C. Needlescopic surgery for broad ligament hernia: A case report (2019) Asian J Endosc Surg 12. https://doi.org/10.1111/ases.12692
- Kopko J, Stańczak R, Warzecha D and Wielgos M. Uterine torsion in the second trimester of pregnancy (2019) Neuro Endocrinol Lett 39: 423-426.
- Padma AM, Truong M, Jar-Allah T, Song MJ, Oltean M, et al. The development of an extended normothermic ex vivo reperfusion model of the sheep uterus to evaluate organ quality after cold ischemia in relation to uterus transplantation (2019) Acta Obstet Gynecol Scand 98: 1127-1138. https://doi.org/10.1111/aogs.13617
- Chen C, Chu HH, Shin JH, Li HL, Ko HK, et al. Inferior mesenteric artery embolization for persistent postpartum hemorrhage after sufficient bilateral iliac arteries embolization: safety and efficacy in eight patients (2019) Br J Radiol 14:20180896. https://doi.org/10.1259/bjr.20180896
- Saat N, Risvanli A, Dogan H, Onalan E, Akpolat N, et al. Effect of melatonin on torsion and reperfusion induced pathogenesis of rat uterus(2019) Biotech Histochem 9:1-7. https://doi.org/10.1080/10520295.2019.1605456
- Tardieu A, Chazelas P, Faye PA, Favreau F, Nadal-Desbarats L, et al. Changes in the metabolic composition of storage solution with prolonged cold ischemia of the uterus (2019) J Assist Reprod Genet 36: 1169–1178. https://doi.org/10.1007/s10815-019-01477-y
- Tardieu A, Dion L, Lavoué V, Chazelas P, Marquet P, et al. The key role of warm and cold ischemia in uterus transplantation: a review (2019) J Clin Med 8: E760. https://doi.org/10.3390/jcm8060760
- Tsompos C, Panoulis C, Toutouzas K, Triantafyllou A, Zografos G, et al. The antioxidant drug u-74389g effect on alanine aminotransferase levels (2017) J Anal Pharm Res 4: 00095. https://doi.org/10.2174/1871529x17666170201104158
*Corresponding author:
Tsompos Constantinos, Department of Gynecology, General Hospital of Thessaloniki St. Dimitrios, 2 Elenis Zografou street, Thessaloniki 54634, Hellas, Greece, Tel: 00302313322171, Fax: 00302106811215, E-mail: Tsomposconstantinos@gmail.com
Citation:
Τsompos C, Panoulis C, Τοutouzas K, Triantafyllou A, Zografos CG, et al. The rat uterus after U-74389g process (2019) Edel J Biomed Res Rev 1: 1-5.
Keywords
Ischemia, U-74389g, Endometrial edema, Endometrial karyorrhexis, Uterus congestion, Uterus inflammation, Reperfusion.


 PDF
PDF