Review Article :
Molecular engineers are studying FAAH as a
target for pharmaceuticals as controlling levels of FAAH may produce some of
the same health effects that excite clinicians about the potential for
phytocannabinoid-based medicines. Synthetic cannabinoids work by flooding the
system with molecules structurally similar to THC and other phytocannabinoids.
Medicines that inhibit the bodys production of FAAH are theorized to have a
similar effect by maximizing the concentration of deficient endocannabinoids in
the nervous system. Technological limitations coupled with a suppression of
research of biologic cannabinoids at many major research universities have
limited our understanding of the endocannabinoid system. Questions still need
to be answered to provide a comprehensive comparison of biologic with synthetic
FAAH inhibitors. Advancement and research aimed at understanding of endogenous
and exogenous cannabinoids, and particularly the medicinal properties of the
Trans-Δ⁹-Tetrahydrocannabinol (THC) molecule and its endocannabinoid equivalent
anandamide are hindered by prohibitive restrictions resulting from the Food and
Drug Administration (FDA), Drug Enforcement Administration (DEA), National
Institute of Health (NIH), and the National Institute on Drug Abuse (NIDA). The
mission statements of each of these entities effectively integrate to ensure
research and utilization of the medicinal properties of THC will be nearly
impossible to attain. Analyzing the advantages of
pharmaceutical as opposed to nutraceutical approaches towards maintaining
health from a biopsychological
perspective tends to become convoluted, particularly with respect to FAAH
inhibitors. This topic pertains to every age group, but typically manifests
itself most dramatically at the age when individuals begin to stop producing
appropriate levels of N-arachidonoyl ethanolamine (Anandamide) and
2-Arachidonoylglycerol (2-AG). Because of individual differences, and varying
degrees of exposure to environments which hasten endocannabinoid depletion, age
of onset varies but usually expresses itself most dramatically around the age
of onset of arthritis, although various ailments and environmental
circumstances can also cause deficiencies in these and other endocannabinoids. All humans possess a measurable
endocannabinoid tone reflecting levels of Anandamide (AEA) and
2-arachidonoylglycerol (2-AG). These have been designated as centrally acting
endocannabinoids, and their decreased concentration shows a significant
correlation to the development of lowered pain threshold, along with
derangements. Autism, ADHD, Parkinsons
disease, Alzheimers disease, Crohns Disease, diabetes, migraines,
fibromyalgia, post-partum depression, muscular dystrophy, multiple sclerosis,
Polyneuropathy, Post Traumatic Stress Disorder, and sleep disorders have all
been implicated in studies as being caused by deficiencies of various
endocannabinoids [1-15]. Endocannabinoid deficiencies can
also arise due to genetic or congenital reasons or are acquired due to
inter-current injury or disease, which consequently produce characteristic
pathophysiological syndromes with symptomatology. Currently, competing
approaches are attempting to emerge as the accepted technique for treating
these endocannabinoid deficiency disorders. Each has advantages and
disadvantages, both biological and psychosocial. This disquisition is designed
to analyze each from a bio-psychological perspective. Pharmaceutical and nutraceutical
approaches to treating endocannabinoid deficiency disorders compete in
remarkable ways, with the former having the advantage of being able to claim
FDA approval. Since its inception, people have been conditioned to believe FDA
approved means safe, although this perception is becoming questioned as adverse
effects of FDA approved medications are increasingly exposed. The latter has
the advantage of being natural, providing it some biomolecular superiority.
Pharmaceuticals have the disadvantage of side-effects, often resulting from the
bodys inability to degrade the synthetic molecules of which they are composed.
Because they are natural, nutraceuticals have the disadvantage of being
unpatentable and therefore cannot be considered for FDA approval. While the concepts discussed in
this paper relate to all forms of endocannabinoid deficiency disorders, only
the deficiency of anandamide will be discussed. Due to ongoing bias against
Trans-Δ⁹-Tetrahydrocannabinol (THC), phytocannabinoid supplementation for this
endocannabinoid deficiency is often eliminated as a nutraceutical approach due
to its potential of producing dopamine in amounts of concern to the National
Institute of Health (NIH), the National Institute of Drug Abuse (NIDA), and
the Drug
Enforcement Administration (DEA). The unstated and somewhat murky
mandate from each of these bureaucratic entities is that researchers devise a
method of increasing N-arachidonoyl ethanolamide (anandamide) levels sans the
Trans-Δ⁹-Tetrahydrocannabinol molecule. THC has been excluded from the 2016
Farm Bill which classified all other phytocannabinoids as agricultural
products, thereby legalizing research of their potential medicinal properties
provided they are derived from varieties of Cannabis sativa that contain less
than 0.3% THC. THC is the most researched of all the phytocannabinoids
throughout the world and its medicinal applications are well-documented, yet
the war on the cannabis plant in the United States is now focused against this
individual phytocannabinoid.
NIDA justifies this war because its ingestion activates the release of
dopamine. Methods of activating dopamine must be legal and socially acceptable.
These methods include religion, jogging, shopping, gambling, video games, and
the ingestion of alcohol, nicotine, and pharmaceutical medications [16]. On June 25th, 2018, the National
Institute of Drug Abuse published on their website their acknowledgment that
the phytocannabinoid equivalent of the endocannabinoid anandamide is
Trans-Δ⁹-tetrahydrocannabinol. While this was not an actual study, the
acknowledgment is a significant step towards the implementation of a
complementary alternative medicine approach in the United States because it
indicates an acceptance of multiple research studies which America funded, but
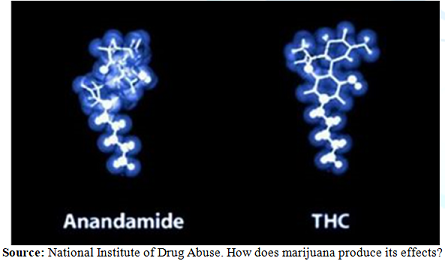
rejected consistently for well over five decades [17] (Figure 1). Figure 1: Anandamid and
Trans-Δ⁹-Tetrahydrocannabinol. Anandamide
is the bodys natural THC molecule possessing multiple medicinal properties,
particularly an ability to relieve neuropathic pain [18]. Inhibition of FAAH
increases endocannabinoid concentrations in both rats and humans providing
therapeutic benefits for virtually every form of endocannabinoid deficiency
disorder [19,20]. Molecular engineers are studying FAAH as a target for
pharmaceuticals because controlling levels of FAAH may produce some of the same
health effects that excite clinicians about the potential for
phytocannabinoid-based medicines. Synthetic cannabinoids work by flooding the
system with molecules structurally similar to THC and other phytocannabinoids.
Medicines that inhibit the bodys production of FAAH are theorized to have a
similar effect by maximizing the concentration of deficient endocannabinoids in
the nervous system. Put simply, if the deficiency is in Anandamide, reduced
FAAH results in more Anandamide availability. While ingestion of
phytocannabinoids increases the number of cannabinoid transmitters artificially
through the addition of THC, the molecule produces dopamine in a federally
unacceptable way. Increasing the concentration of
endocannabinoids by inhibiting FAAH and other catabolic enzymes, rather than
administering exogenous agents is theorized to reduce cannabinoid-like adverse
events attributed to intromission of phytocannabinoids [21]. Synthetic FAAH
inhibitors exhibit neurological side effects not manifested by the biologic,
including impairment of cognition and motor functions and a predisposition to
psychoses, notably when these agents are used for long-term treatment [22]. The development of potent and
safe synthetic FAAH
inhibitors has been hindered by their deleterious side effects [23]. On
July 9, 2015, Biotrial, a Contract Research Organization began human phase
testing of the synthetic FAAH inhibitor BIA 10-2474 for the manufacturer by
recruiting 128 healthy volunteers, both men and women aged 18 to 55. The study
employed a three-stage design with 90 of the volunteers receiving the drug
during the first two stages of the trial, with no serious adverse events
reported. Participants of the study were asked to stay at Biotrials facility
for two weeks, during which time they would take the drug for ten days and
undergo tests. In the third stage of the trial
evaluating multiple doses, six male volunteers received doses by mouth,
starting on 7 January 2016. The first volunteer was hospitalized on January 10,
became brain dead, and died on January 17. The other five men in the same
dosage group were also hospitalized from January 10 through January 13, four of
them suffering injuries, including deep hemorrhagic and necrotic lesions seen on
brain MRI. Professor Pierre-Gilles Edan, a neurologist at the University of
Rennes Hospital Center, stated in a press conference that three of the four men
were displaying neurological symptoms severe enough to create a clinical
picture to fear that even in the best situation there will be an irreversible
handicap. The experiment was discontinued on January 11, 2016 [24]. Many
questions remain unanswered, including the biomolecular mechanism causing the
participants injuries. Magnetic-resonance-imaging scans revealed dying and
bleeding tissue deep in the brain. The devastating result of this
clinical trial led to a scramble of scientists proposing various explanations
as to the cause of the deadly side-effect resulting from the synthetic FAAH
inhibitor. It has been suggested that the adverse events may come from its
binding to unidentified off-targets. However, few methods exist to predict
cellular off-target effects resulting from the drug binding to biological
assemblies, and their associations with diseases [25]. Owing to these
limitations, it is still unclear what the off-targets of FAAH inhibitors are,
and how the off-target affects the system-level response [26]. FAAH inhibitors are designed to remove fatty
acid amide hydrolase proportionally, thereby increasing the concentration of
anandamide naturally produced by the body. While synthetic FAAH inhibitors have
been demonstrated to do this, we now know enough about both the endocannabinoid
system and biomolecular psychology to theorize about the mechanism by which
synthetic compounds cause neurological damage [27]. The adverse effects are
likely not a byproduct of FAAH-inhibition directly, but rather the result of
biologic enzymes being incapable of effectively degrading them. Biologic FAAH
inhibitors demonstrate significant differences in their molecular structures
than their synthetic counterparts, and the differences in the molecular
structures may account for differences in the safety profiles between the
synthetic and the biologic. These differences could be related to the time it
takes the FAAH inhibitors to degrade. Information is lacking about what enzyme
degrades either synthetic or biologic FAAH inhibitors, and this is an area
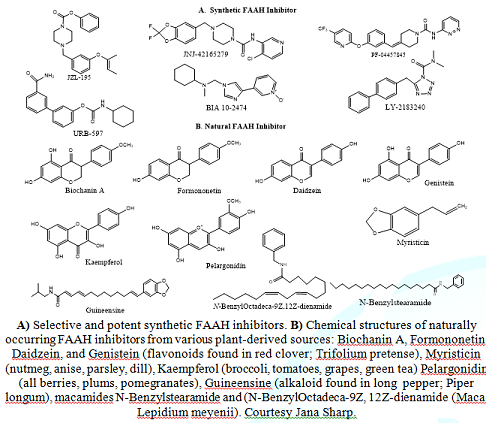
where further research is warranted (Figure 2). Figure 2: Chemical structures of Synthetic and
Naturally Occurring FAAH Inhibitors. Technological limitations,
coupled with a suppression of research of biologic cannabinoids at many major
research universities, has resulted in a limited understanding of the
endocannabinoid system. Questions still need to be answered to provide a
comprehensive comparison of biologic with synthetic FAAH inhibitors. An
exhaustive review of the literature provides no definitive explanation as to
which natural enzyme degrades the biologic and synthetic inhibitors. Monoacylglycerol
lipase (MAG) appears to be one likely culprit, but further research is
needed in this area [28,29]. A determination of the enzyme is
necessary to design an in vitro study to verify the theory that there is a
significant difference in degradation rates between synthetic and biologic FAAH
inhibitors. A difference in these degradation rates would explain the
differences in adverse events exhibited in the synthetic and biologic FAAH
inhibitors. Although the science concerning the efficacy of supplementing
phytocannabinoids to treat deficiencies of endocannabinoids is robust and
well-accepted, utilization of this knowledge is still in its beginning stages
[30-32]. Technological advancement and
research aimed at understanding endogenous and exogenous cannabinoids, and
particularly the medicinal properties of the Trans-Δ⁹-tetrahydrocannabinol
(THC) molecule, are hindered by prohibitive restrictions resulting from the
mission statements of the Food and Drug Administration (FDA), Drug Enforcement
Administration (DEA), National Institute of Health (NIH), and the National
Institute on Drug Abuse (NIDA). The missions of these entities
effectively integrate to ensure research and utilization of the medicinal
properties of THC face stiff resistance. One of the mandates of the FDA is to
evaluate any medicine submitted to it provided the medicine has a synthetic
(patentable) component. The mission of the NIDA is to advance science on the
causes and consequences of drug use and addiction and to apply that knowledge
to improve individual and public health. NIH through NIDA has provided and
continues to provide funding for studies related to therapeutic uses of
cannabinoids, including THC as it pertains to its mission, but the vast
majority of research proposals funded involve therapeutic benefits of
individual phytocannabinoids and not the utilization of an entourage of these
molecules to measurably manipulate endocannabinoid tone. NIDA predominantly
funds research on the use of individual molecules due to the difficulty of
standardizing dosing with full-plant preparations. As the federal agency
responsible for determining which cannabinoid studies get funded and what
questions remain unanswered, NIDA traditionally restricts this research to the
deleterious effects of phytocannabinoid ingestion, particularly focusing on
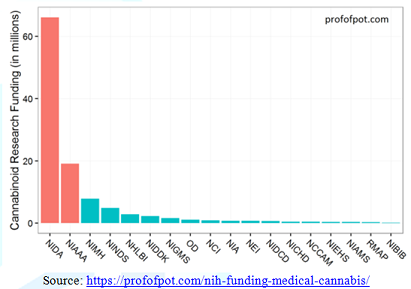
dopamine-releasing actions of the THC molecule. NIH admits that only 19% of their
research funds are slated to studying the possible therapeutic properties of
phytocannabinoids, and this is with admittedly a very loose interpretation of
the definition of therapeutic (National Institute of Health, 2018). In fiscal
year 2015, NIH supported 281 projects totaling over $111 million dollars on
cannabinoid research, with this funding disproportionately slated to the two
Institutes with stated missions of designing studies for the purpose of
exposing purported negative health effects of intromitting phytocannabinoids:
The National Institute on Drug Abuse and the National Institute on Alcohol
Abuse and Alcoholism [33] (Figure 3). The Drug
Enforcement Administration (DEA) was created in 1973 to enforce criminal
penalties on individuals for using unapproved exogenous compounds to increase
their dopamine levels. Unsurprisingly, the conglomeration of the missions of
these four entities has the US federal government focusing much more on
researching the negative effects of phytocannabinoids rather than their
well-documented medicinal properties. As of September 1, 2019, every
State except Nebraska allows their residents to medicate with
phytocannabinoids, with 14 of these regulating the percentage of THC. Some
Idaho, South Dakota, and Indiana residents have attained access after
successfully challenging their state bureaucracies. Because of this and overwhelming
public acknowledgment of the efficacy of medicinal cannabis, the general
perception of the population is that federal acceptance of medicinal cannabis
is imminent, but unless the application of the mission statements of the four
federal agencies involved change, science must develop alternate approaches for
modulating the endocannabinoid system. Even with the possible acceptance of the
CBD molecule due to the erroneous claim that it lacks psychoactive properties,
the utilization of the medicinal properties of THC conflict with the mission
statements of each of the four bureaucratic entities that have a say in the
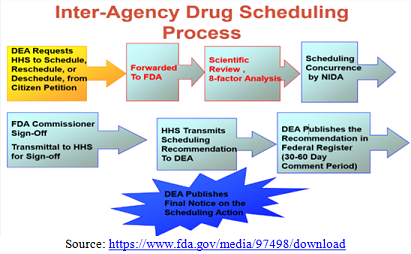
decision concerning its legalization at the federal level [34,35] (Figure 4). Figure 4: Inter agency drug scheduling process. By mandate, unless the
utilization of medicinal cannabis is legalized at the federal level, the
therapeutic properties of the exogenous THC phytocannabinoid must come from
increasing levels of its endogenous equivalent, anandamide. The FDA is
currently working with pharmaceutical companies to establish the appropriate
path forward for the synthesis of safe and effective FAAH inhibitors, but human
clinical trials for these drugs are many years and many billions of dollars in
the future [36]. Until the four bureaucratic agencies revise their missions to
allow for the utilization of the exogenous cannabinoid the medicinal benefits
inherent in THC must occur by scientists devising efficacious methods of
increasing the concentration of its endogenous equivalent. Biochanin
A is an isoflavone mainly found in red clover. It has poor solubility and
oral absorption and exhibits various effects, including anti-inflammatory,
estrogen-mimicking, and glucose lipid modulatory activity, as well as being a
cancer preventive, and neuroprotectant [37-48]. It is already commercially
available and among the main ingredients in many types of supplements used to
alleviate postmenopausal symptoms in women. In addition to these benefits,
Biochanin A is a mixed-type inhibitor of FAAH, demonstrating low micromolar
potencies towards rat, mouse, and recombinant human FAAH, sans the adverse effects
so commonly associated with its synthetic counterparts. It has drawn
considerable attention from researchers in recent years owing to the wide
spectrum of its pharmacological activity, many related to its actions as a
natural inhibitor of fatty acid amide hydrolase. FAAH is the enzyme responsible
for the metabolism (degradation) of the endogenous cannabinoid receptor ligand
anandamide (AEA) and many other endogenous fatty acid amides, exhibiting a
distribution consistent with its role in regulating (terminating) their effects
at their released sites of action. This action provides the
mechanism responsible for the effectiveness Biochanin A exhibits in treating
multiple endocannabinoid deficiency disorders including Post
Traumatic Stress Disorder, Autism, ADHD, Alzheimers disease, Multiple
Sclerosis, Dementia, Parkinsons disease, Huntingtons disease, and scores of
other nervous system disorders resulting from deficiencies in anandamide
[49-52]. Thors et al. investigated a
series of analogs of the isoflavones genistein and daidzein to provide
illumination on the structural requirements for FAAH inhibition and to
determine whether more potent natural analogs could be found. Among the analogs
tested, biochanin A, was shown to be a more potent inhibitor of FAAH than
genistein in vitro, and to produce biochemical effects upon a spinal cord pain
signaling pathway consistent with FAAH inhibition in vivo without the adverse
effects of synthetic FAAH inhibitors [53]. Biochanin A has drawn the
considerable attention of researchers in recent years due to its wide array of pharmacological
actions including its neuroprotective, anticancer, antioxidant,
anti-inflammatory, osteogenic, and anti-hyperglycemic properties. Even though
the therapeutic potential of this isoflavone is intriguing and has been studied
in a variety of in vitro, in vivo and ex vivo models, its potential has been
deemed limited due to its low oral bioavailability. As is often the case in
scientific endeavors related to biomolecular psychology, an innovative approach
must be devised to adapt to identified limitations. Biochanin A is a poorly
soluble bioflavonoid, and this characteristic prevents its oral absorption.
While ingestion is typical for nutraceuticals, a more innovative method of
intromission must be developed. Creativity is the essence of the scientific
process, and new methods of intromission of medicines which increase
bioavailability are constantly being devised. Transdermal patches deliver a
specific dose of medication into the bloodstream through a porous membrane. An
advantage of a transdermal delivery route is that a patch provides a controlled
release of the compound into the subject. A wide variety of pharmaceuticals are
now available in transdermal patch form, and this delivery method can easily be
appropriated to enhance the bioavailability of nutraceuticals such as Biochanin
A [54]. Psychosocial, political, and
bureaucratic policies dictate much of the US landscape of research into
endogenous and exogenous cannabinoids, particularly THC. Researchers are
identifying endocannabinoid deficiency disorders and mechanisms through which
treatment approaches may be developed. Endocannabinoid
deficiency disorders may be effectively treated through the supplementation
of equivalent phytocannabinoids. Molecular engineers are studying FAAH as a
target for pharmaceuticals as controlling levels of FAAH may produce some of
the same health effects that excite clinicians about the potential for
phytocannabinoid-based medicines. Medicines that inhibit the bodys production
of FAAH are theorized to have a similar effect by maximizing the concentration
of deficient endocannabinoids endogenously, but the development of potent and
safe synthetic FAAH inhibitors has been hindered by their deleterious side
effects. Differences in the bodys ability to metabolize synthetic and biologic
FAAH inhibitors are theorized to contribute to their differing safety profiles,
but the biomolecular degradation mechanism remains unidentified and is an area
where further research is warranted. 1.
Chiurchiù V, Van der Stelt, M,
Centonze D and Maccarrone M. The endocannabinoid system and its therapeutic
exploitation in multiple sclerosis: Clues for other neuroinflammatory diseases
(2018) Progress in Neurobiology 160: 82-100. https://doi.org/10.1016/j.pneurobio.2017.10.007
2.
Clark TM, Jones JM, Hall AG,
Tabner SA and Kmiec RL. Theoretical explanation for reduced body mass index and
obesity rates in cannabis users (2018) Cannabis and Cannabinoid Research 3:
259-271. https://doi.org/10.1089/can.2018.0045
3. Fezza
F, Bari M, Florio R, Talamonti E, Feole M, et al. Endocannabinoids, related
compounds and their metabolic routes (2014) Molecules 19: 17078-17106. https://doi.org/10.3390/molecules191117078
4. Karhson
DS, Krasinska KM, Dallaire JA, Libove RA, Phillips JM, et al. Plasma anandamide
concentrations are lower in children with autism spectrum disorder (2018)
Molecular autism 9. https://doi.org/10.1186/s13229-018-0203-y
5. Krumbholz
A, Anielski P, Reisch N, Schelling G and Thieme D. Diagnostic value of
concentration profiles of glucocorticosteroids and endocannabinoids in hair
(2013) Therapeutic Drug Monitoring 35: 600-607. https://doi.org/10.1097/FTD.0b013e3182953e43
6. Kuhathasan
N, Dufort A, MacKillop J, Gottschalk, R, Minuzzi, L, et al. The use of
cannabinoids for sleep: A critical review on clinical trials (2019)
Experimental and Clinical Psychopharmacology 27: 383-401. https://doi.org/10.1037/pha0000285
7. Martínez-Pinilla
E, Aguinaga D, Navarro G, Rico A J, Oyarzábal J, et al. Targeting CB1 and GPR55
endocannabinoid receptors as a potential neuroprotective approach for
Parkinsons Disease (2019) Molecular Neurobiology 58: 5900-5910. https://doi.org/10.1007/s12035-019-1495-4
8.
Mbachi C, Attar B, Wang Y, Paintsil I, Mba B, et al. Association between
cannabis use and complications related to Crohns Disease: A retrospective
cohort study (2019) Digestive Diseases and Sciences 64: 2939-2944. https://doi.org/10.1007/s10620-019-05556-z
9. Nazıroğlu
M, Taner AN, Balbay E and Çiğ, B. Inhibitions of anandamide transport and FAAH
synthesis decrease apoptosis and oxidative stress through inhibition of TRPV1
channel in an in vitro seizure model (2019) Molecular and Cellular Biochemistry
453: 143-155. https://doi.org/10.1007/s11010-018-3439-0
10. Neumeister
A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, et al. Elevated brain
cannabinoid CB1 receptor availability in post-traumatic stress disorder: a
positron emission tomography study (2013) Molecular Psychiatry 18: 1034-1040. https://doi.org/10.1038/mp.2013.61
11. Pál
P and George K. Modulating the endocannabinoid system in human health and disease
– successes and failures (2013) The FEBS Journal 280: 1918-1943. https://doi.org/10.1111/febs.12260
12. Russo
EB. Clinical Endocannabinoid Deficiency Reconsidered: Current Research supports
the theory in migraine, fibromyalgia, irritable bowel, and other treatment-resistant
syndromes (2016) Cannabis and Cannabinoid Research 1: 154-165. https://doi.org/10.1089/can.2016.0009
13. Smith
SC and Wagner MS. Clinical endocannabinoid deficiency (CECD) revisited: Can
this concept explain the therapeutic benefits of cannabis in migraine,
fibromyalgia, irritable bowel syndrome and other treatment-resistant
conditions? (2014) Neuro Endocrinology Letters 35: 198-201. 14. Stumm
C, Hiebel C, Hanstein R, Purrio M, Nagel H, et al. Cannabinoid receptor 1
deficiency in a mouse model of Alzheimers disease leads to enhanced cognitive
impairment despite of a reduction in amyloid deposition (2013) Neurobiology of
Aging 34: 2574-2584. https://doi.org/10.1016/j.neurobiolaging.2013.05.027
15. Wilker
S, Pfeiffer A, Elbert T, Ovuga E, Karabatsiakis A, et al. Endocannabinoid
concentrations in hair are associated with PTSD symptom severity (2016)
Psychoneuroendocrinology 67: 198-206. https://doi.org
/10.1016/j.psyneuen.2016.02.010 16. Lustig
R. The Hacking of the American Mind: The Science Behind the Corporate Takeover
of Our Bodies and Brains New York (2018) Penguin Publishers, USA. 17. NIDA.
(2019) Marijuana: How does marijuana produce its effects?
18. Bhuniya
D, Kharul RK, Hajare A, Shaikh N, Bhosale S, et al. Discovery and evaluation of
novel FAAH inhibitors in neuropathic pain model (2019) Bioorganic and Medicinal
Chemistry Letters 29: 238-243. https://doi.org/10.1016/j.bmcl.2018.11.048
19. Ahn
K, Smith SE, Liimatta MB, Beidler D, Sadagopan N, et al. Mechanistic and
pharmacological characterization of PF-04457845: a highly potent and selective
fatty acid amide hydrolase inhibitor that reduces inflammatory and
noninflammatory pain (2011) The Journal of Pharmacology and Experimental
Therapeutics, 338: 114-124. https://doi.org/10.1124/jpet.111.180257
20. Li
GL, Winter H, Arends R, Jay GW, Le V,et al. Assessment of the pharmacology and
tolerability of PF-04457845, an irreversible inhibitor of fatty acid amide hydrolase-1,
in healthy subjects (2012) British Journal of Clinical Pharmacology 73: 706-716.
https://doi.org/10.1111/j.1365-2125.2011.04137.x
21. Ahn
K, McKinney MK and Cravatt BF. Enzymatic pathways that regulate endocannabinoid
signaling in the nervous system(2008) Chemical Reviews 108: 1687-1707. https://doi.org/10.1021/cr0782067
22. Eva
MS, Toshiaki AF, JulianT and Jose Luis RM. Systematic review and meta-analysis
of cannabis treatment for chronic pain (2009) Pain Medicine 8: 1353. https://doi.org/10.1111/j.1526-4637.2009.00703.x
23. Butler
D and Callaway E. Scientists in the dark after French clinical trial proves
fatal (2016) Nature 529: 263-264. https://doi.org
/10.1038/nature.2016.19189 24. Kaur
R, Sidhu P and Singh S. What failed BIA 10-2474 Phase I clinical trial? Global
speculations and recommendations for future Phase I trials (2016) Journal of
Pharmacology & Pharmacotherapeutics, 7: 120-126. https://doi.org/10.4103/0976-500X.189661
25. Dider
S, Ji J, Zhao Z and Xie L. Molecular mechanisms involved in the side effects of
fatty acid amide hydrolase inhibitors: a structural phenomics approach to
proteome-wide cellular off-target deconvolution and disease association (2016) NPJ
Systems Biology and Applications 2: 16023. https://doi.org/10.1038/npjsba.2016.23
26. Mallet
C, Dubray C and Dualé C. FAAH inhibitors in the limelight, but regrettably
(2016) International Journal of Clinical Pharmacology and Therapeutics 54: 498-501.
https://doi.org/10.5414/CP202687
27. Dawson
DA. Synthetic cannabinoids, organic cannabinoids, the endocannabinoid system,
and their relationship to obesity, diabetes, and depression (2018) Journal of
Molecular Biology 7: 219. 28. Bononi
G, Granchi C, Lapillo M, Giannotti M, Nieri D, et al. Discovery of long-chain
salicylketoxime derivatives as monoacylglycerol lipase (MAGL) inhibitors (2018)
European Journal of Medicinal Chemistry 157: 817-836. https://doi.org/10.1016/j.ejmech.2018.08.038
29. Pertwee
RG. Elevating endocannabinoid levels: pharmacological strategies and potential
therapeutic applications (2014) The Proceedings of The Nutrition Society 73:
96-105. https://doi.org/10.1017/S0029665113003649
30. Hill
MN, Campolongo P, Yehuda R and Patel S. Integrating endocannabinoid signaling
and cannabinoids into the biology and treatment of posttraumatic stress
disorder (2018) Neuropsychopharmacology 43: 80-102. https://doi.org/10.1038/npp.2017.162
31. Morena
M, Patel S, Bains JS and Hill MN. Neurobiological interactions between stress
and the endocannabinoid system (2016) Neuropsychopharmacology 41: 80-102. https://doi.org/10.1038/npp.2015.166
32. Ney
LJ, MatthewsA, Bruno R and Felmingham KL. Cannabinoid interventions for PTSD:
Where to next? (2019) Progress in Neuropsychopharmacology & Biological
Psychiatry 93: 124-140. https://doi.org
/10.1016/j.pnpbp.2019.03.017 33. Emery
MS. (2016) What Is NIH Funding of Therapeutic Cannabinoid Research Really For?
34. Dawson
DA. Cannabidiol Psychoactivity: A perspective on claims of US Patent 6630507
(2019) Journal of Medical Biomedical and Applied Sciences 7: 200-201. https://doi.org/10.15520/jmbas.v7i1.174
35. Russo
EB. Cannabidiol claims and misconceptions (2017) Trends in Pharmacological
Sciences 38: 198-201. https://doi.org/10.1016/j.tips.2016.12.004
37. Peterson
G and Barnes S. Genistein and biochanin A inhibit the growth of human prostate
cancer cells but not epidermal growth factor receptor tyrosine
autophosphorylation (1993) Prostate 22: 335-345. https://doi.org/10.1002/pros.2990220408
38. Raheja
S, Girdhar A, Lather V and Pandita D. Biochanin A: A phytoestrogen with
therapeutic potential (2018) Trends in Food Scie and Tech 79: 55-66. https://doi.org/10.1016/j.tifs.2018.07.001
39. Rossi
G, Gasperi V, Paro R, Barsacchi, D, Cecconi S, et al. Follicle-stimulating
hormone activates fatty acid amide hydrolase by protein kinase A and
aromatase-dependent pathways in mouse primary Sertoli cells(2007)
Endocrinology, 148: 1431-1439. 40. Sehdev
V, Lai JCK and Bhushan A. Biochanin a modulates cell viability, invasion, and
growth promoting signaling pathways in her-2-positive breast cancer cells
(2009) J of Oncology 2009: 121458. https://doi.org/10.1155/2009/121458
41. Sehm
T, Fan Z, Weiss R, Schwarz M, Engelhorn T, et al. The impact of dietary
isoflavonoids on malignant brain tumors (2014) Cancer Med 3: 865-877. https://doi.org/10.1002/cam4.265
42. Sun
XY, Plouzek CA, Henry JP, Wang TT and Phang JM. Increased UDP-glucuronosyltransferase
activity and decreased prostate specific antigen production by biochanin A in
prostate cancer cells (1998) Cancer Res58: 2379-2384. 43. Tan
JW and Kim MK. Neuroprotective effects of biochanin a against β-amyloid-induced
neurotoxicity in pc12 cells via a mitochondrial-dependent apoptosis pathway
(2016) Molecules 21: 548. https://doi.org/10.3390/molecules21050548
44. Wang
J, Wu WY, Huang H, Li WZ, Chen HQ, et al. Biochanin A protects against
lipopolysaccharide-induced damage of dopaminergic neurons both in vivo and in
vitro via inhibition of microglial activation (2016) Neurotoxicity Res 30: 486-498.
https://doi.org/10.1007/s12640-016-9648-y
45. Xiao
P, Zheng B, Sun J and Yang J. Biochanin A induces anticancer effects in SK-Mel-28
human malignant melanoma cells via induction of apoptosis, inhibition of cell
invasion and modulation of NF-κB and MAPK signaling pathways (2017) Oncology
Letters 14: 5989-5993. https://doi.org/10.3892/ol.2017.6945
46. Yanagihara
K, Numoto M, Tauchi H, Akama Y, Yokozaki H, et al. Genetic status of p53 and
induction of apoptosis by radiation or isoflavones in human gastric carcinoma
cell lines (1996) International Journal of Oncology 9: 95-102. 48. Zhao
X, Tang X, Guo N, An Y, Chen X, et al. Biochanin A enhances the defense against
salmonella enterica infection through AMPK/ULK1/mTOR-Mediated Autophagy and
Extracellular Traps and Reversing SPI-1-Dependent Macrophage (MΦ) M2
Polarization (2018) Frontiers in Cellular and Infection Microbiology 8: 318. https://doi.org/10.3389/fcimb.2018.00318
49. Ahn
K, Johnson DS and Cravatt BF. Fatty acid amide hydrolase as a potential
therapeutic target for the treatment of pain and CNS disorders (2009) Expert
Opinion on Drug Discovery 4: 763-784. 50. Bari
M, Battista N, Valenza M, Mastrangelo N, MalapontiM, et al. In vitro and in
vivo models of Huntingtons disease show alterations in the endocannabinoid
system(2013) FEBS Journal 280: 3376-3388. https://doi.org/10.1111/febs.12329
51. Filippo
DM, Pini LA, Pellicciolz GP, Calabresi P and Sarchielli P. Abnormalities in the
cerebrospinal fluid levels of endocannabinoids in multiple sclerosis (2008)
Journal of Neurology, Neurosurgery, And Psychiatry 79: 1224-1229. https://doi.org/10.1136/jnnp.2007.139071
52. Zou
M, Li D, Li L, Wu L and Sun C. Role of the endocannabinoid system in
neurological disorders (2019) Int J of Devel Neurosci 76: 95-102. https://doi.org
/10.1016/j.ijdevneu.2019.03.002 53. Thors
L, Burston JJ, Alter BJ, McKinney MK, Cravatt BF, et al. Biochanin A, a
naturally occurring inhibitor of fatty acid amide hydrolase (2010) British Jof
Pharm 160: 549-560. https://doi.org/10.1111/j.1476-5381.2010.00716.x
54.Patel
AV and Shah BN. Transdermal drug delivery system: A review (2018) Pharma Sci
Monitor 9: 378-390. David
A Dawson, Endocannabinoid Specialist, 2456 Ecuadorian Way, Suite 34,
Clearwater, FL, USA, Tel: 33763 458-229-2021, E-mail: d.dawson8352@ncu.edu Dawson
DA. The psychosocial and biological aspects of synthetic and natural FAAH
inhibitors (2019) Edel J Biomed Res Rev 1: 6-11. FAAH inhibitors, Endocannabinoid System, Endocannabinoids,
Phytocannabinoids, Anandamide, Pharmaceuticals, Nutraceuticals, Biochanin A.Psychosocial and Biological Aspects of Synthetic and Natural FAAH Inhibitors
David A Dawson
Abstract
Full-Text
Introduction
Psychosocial Aspects of
Pharmaceutical and Nutraceutical Approaches to Healthcare
Anandamid and Trans-Δ⁹-Tetrahydrocannabinol
Adverse Effects of Synthetic FAAH
Inhibitors
Degradation of Synthetic and
Biologic FAAH inhibitors
A Psychosocial Perspective of the
Endocannabinoid System
A Natural Fatty Acid Amide
Hydrolase Inhibitor
Issues of Bioavailability
Summary
References
*Corresponding author:
Citation:
Keywords