Introduction
Zinc homeostasis is a key factor
in maintaining a healthy
immune system. Zinc ions are involved in regulating intracellular signaling
pathways in innate and adaptive immune cells that the influences of zinc status
on the overall immune function are present in zinc deficiency as overproduction
of pro-inflammatory cytokines and reactive mediators, zinc homeostasis as
balanced immune cell functions and zinc excess as suppression of T and B cell
functions [1]. Zinc is known to be essential for highly growth and development
of all organisms in the human body, especially the immune system. A variety of
effects of zinc on immune cells depend on the zinc concentration that in a
concentration of 100 μmol/L, zinc suppresses natural killer cell killing and
T-cell function whereas monocytes are activated directly and in a concentration
of 500 μmol/L, zinc evokes a direct chemotactic activation of neutrophil granulocytes
[2]. Zinc is a fundamental trace element in human body that the recommended daily
intake of zinc depends on several factors. Average values of recommended intake
may be 7~11 mg/day for
adults. Zinc is the second abundant trace metal with human body 2~3 g and a
plasma concentration of 12-16 μM, 90% in muscle and bone and 10% other organs
include prostate, liver, the gastrointestinal tract, kidney, skin, lung brain,
heart and pancreas in humans that cellular zinc underlies an efficient
homeostatic control that avoids accumulation of zinc in excess. Zinc status
play an important role in antiviral immunity, mainly during the early stage of
the infection that the most effective antiviral antibodies are neutralizing
antibodies which bind to the viral envelope or capsid proteins and regulate the
virus entering into host cell [3]. Zinc deficiency accounts currently for
approximately 16% of lower respiratory tract infections, 18% of malaria and 10%
of diarrheal diseases, while severe zinc deficiency is rare, mild to moderate
deficiency is more common worldwide [4]. The zinc deficiency leads to
cell-mediated immune dysfunctions among other manifestations which such
dysfunctions lead to a worse outcome in the response towards virus infection
[4]. Zinc homeostasis during acute phase response is the temporal transfer of
serum zinc to the tissues, causing transient serum hypozincemia.
Zinc homeostasis is rebalanced
during resolution of the inflammatory response that intracellularly increased
zinc can intoxicate engulfed pathogens and acts cytoprotective by promotion of
neutralizing Reactive
Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) [4]. The other,
zinc deficiency in Chronic
Kidney Disease (CKD) patients may be due to fecal excretion or decrease in
its absorption that zinc concentrations were lower in Hemodialysis (HD)
patients compared to controls and Zn concentration 69.16 μg/dL of blood in HD
patients, however, revealed no correlation among serum Zn concentration and
anemia, serum parathyroid hormone concentration or pruritus severity in HD
patients [5]. The role of zinc in cell death has apoptosis that the influence
of zinc on apoptosis is tissue/cell type, zinc concentration and expression of
zinc transporters and zinc-binding proteins.
Host zinc homeostasis changes in
response to viral infections, including production of metal sequestering
proteins and bombardment with toxic level of zinc at host-pathogen interface
[6]. Zinc influences apoptosis by acting on several molecular regulators of programmed
cell death and zinc deficiency caused by malnutrition and foods with low
bio-availability, aging, certain diseases and deregulated homeostasis is a far
more common risk to human health without intoxication [7]. Apoptosis is defined
as cell death activated by an internally controlled suicide program that
bacteria are able to trigger apoptosis, including the secretion of compounds
such as protein synthesis inhibitions, pore forming proteins, molecules
responsible for the activation of the endogenous death in the infected cell and
super antigens [8].
The influence of zinc on
apoptosis is very complex that variables in this complex network are tissue and
cell type, zinc concentration, expression of zinc
transporters and zinc-binding proteins, oxidative or nitrosative stress and
the improvement of molecular opposing functions. Regulation of apoptosis is
essential for normal embryonic development and for homeostasis in adult tissue.
Zinc has a rather low toxicity and influences apoptosis by acting on several
molecular regulators of programmed cell death which can inhibit apoptosis
thereby either prolonging the survival of infected cells. Viruses are obligate
intracellular parasites that cause infection by invading cells of the body.
Their life cycle comprises a short extracellular period and a longer
intracellular period during which they undergo replication.
The immune system has
non-specific and specific mechanism that attack the virus in both phases of its
life cycle which specific antibodies protect against viral infections and play
an important role in antiviral immunity, mainly during the early stage of the
infection [9]. Human
coronaviruses (HCoVs) are known as respiratory pathogens that HCoVs play
only a minor role in causing gastrointestinal illness in children<6 year old
which interest in coronaviruses in relation to enteric diseases in humans
increased with the emergence of Severe
Acute Respiratory Syndrome (SARS) and identification of SARS Coronavirus in
2003 [10]. The emergent development of antiviral drugs for new type-coronavirus
(2019-novel CoV) respiratory infection is nowadays desired to be due to the
employment. In this review, the zinc-mediated antiviral immunity and the
virucidal activities of zinc-finger protein, zinc-finger antiviral protein and
zinc-binding domain are discussed against many infectious viruses. Thereby, the
virucidal mechanisms by zinc ions-binding formation, RNA virus degradation and
Zn2+-centered coordination via the zinc-finger antiviral proteins,
zinc-binding domain and zinc-conjugated complexes may be clarified.
Zinc-Induced
Antiviral Immunity
Zinc is an essential trace
element that is crucial for growth, development and the maintenance of immune
function which zinc status is a critical factor that can influence antiviral
immunity, particularly as zinc-deficient populations are often most at risk of
acquiring viral infections such as HIV, HCV [3]. In immune cells, HIV infection
is sensed by several Pattern Recognition Receptors (PRRs), leading to Type 1
Interferon (IFN-1) and inflammatory cytokines production that up regulate antiviral
Interferon-Stimulated Genes (ISGs) [11]. Tripartite Motif (TRIM) 25 enabled
to regulate antiviral innate immunity can bind to RNA, leading to uncover new
mechanism by which this molecule regulates intracellular signaling and/or RNA
virus replication [12]. Common features possess that enveloped viruses enter
cells by membrane-fusion protein on the surface, fusion glycoprotein on
metastable prefusion and interactions with neutralizing antibodies.
Implications for immunogen design of next-generation vaccines have been shown
from the results that stable immunogens presenting the same antigenetic sites
as the labile wild-type proteins efficiently elicit potently neutralizing
antibodies [13].
Zinc-Finger
Protein
Interferon
Induced Transmembrane Proteins (IFITMs) inhibit the cellular entry of a
broad range of viruses that IFITM-mediated restriction requires recognition of
viral RNA elements, in which the IFITMs can inhibit the viral entry of IAV (Influenza
A Virus), HCV, Ebora virus, SARS Coronavirus, Dengue virus, Zika virus and
HIV-1 [14]. In addition, interferon-stimulated genes serve as enhancers of
antiviral innate immunity [15]. The novel EBV-induced Zinc Finger Gene (ZNFEB)
including its intronless locus and human protein variants, controls entry and
exit from cell cycling in activated lymphocytes [16].
The designed polydactyl Zinc-Finger
Protein (ZNF) is prepared consisting HIV-1 type integrase fused to the
synthetic zinc finger protein E2C that the integrase-E2C fusion proteins offer
an efficient approach and a versatile framework for directing the integration
of retroviral DNA into a predetermined DNA site [17]. The ZNF ZCCHC3 binds RNA
and facilitates viral RNA that ZCCHC3 is a co-receptor for the Retinoic
Acid-Inducible Gene-1 (RIG-1) and antigen MDA5 which is critical for RIG-1 like
receptor (RLR)-mediated innate immune response to RNA virus [18]. Artificial
ZFNs strongly block both Sp1-cyclin T1-dependent transcription and Tat-dependent
transcription of HIV-1 [19]. ZNF Tsip1 that the candidate genes encoded
Tsi1‐interacting protein 1 (Tsip1), a ZNF Tsip1 strongly interacted with CMV 2a
protein, controls Cucumber Mosaic Virus (CMV) RNA replication [20].
Zinc-Finger
Antiviral Protein
Zinc-Finger
Antiviral Protein (ZAP) controls
virus entry, DNA/RNA replication and spreading against viral infection. ZAP
specifically inhibits the replication of certain viruses and promotes viral RNA
degradation [21]. ZAP may regulate DNA and RNA virus replication. Inhibition of
bacterial DNA replication during nitrosative stress is accompanied by zinc
mobilization [22]. ZAP inhibits Retroviral RNA production [23] and ZAP inhibits
HIV-1 infection by promoting the degradation of specific viral mRNAs [24]. The
ZAP in first steps of HCV infection may be used as entry inhibitor [25]. ZAP
inhibits alpha virus replication that elucidation of the antiviral mechanism by
which ZAP inhibits Sindbis Virus (SINV) translation may lead to the development
of agents with broad activity against alpha viruses [26]. The ZAP also inhibits
IAV protein expression, in which suggests an important role of ZAP in the host
effort to control IAV infection and the importance of the threat of ZAP to the
virus [27]. The host cell restriction factors that limit IAV have been
investigated [28].
Hence, ZAPs inhibit viral entry,
DNA/RNA replication and spreading that ZAP regulates virus infection with
degradation of specific viral mRNA. Furthermore, this ZAP could probably
inhibit the HCoVs that to date, the six known HCoVs have been identified,
namely HCoV-229E, HcoV-NL63, HCoV-OC43, HCoV-HKU1, SARS-CoV and Middle
East respiratory syndrome corona-virus (MERS-CoV), subsequent phylogenetic
studies pointed to the bat origin of SARS-CoV based on sequences of SARS-like
virus found in bats [29]. Replication of SARS-CoV requires proteolytic
processing of the replicase polyprotein by two viral cystein proteases, a
chymotrypsin-like protease (3CLpro) and a Papain-Like Protease (PLpro).
This PLpro is important for
development of antviral drug that would inhibit viral replication and reduce
mortality associated with outbreaks of SARS-CoV that a model of PLpro in
complex with ubiquitin aldehyde reveals well defined sites within the catalytic
cleft that help to account for strict substrate-recognition motifs [30]. The
MERS-CoV PLpro Blocking Loop 2 (BL2) structure differs from that of SARS-CoV
PLpro, where it has been proven to play a crucial role in SARS-CoV PLpro
inhibitor binding that inhibitor recognition specificity of MERS-CoV PLpro may
differ from that of SARS-CoV PLpro. In addition, inhibitory activity, of this
compound was selective for SARS-CoV and MERS-CoV PLpro enzymes over two human
homologues and the ubiquitin C-terminal hydrolases [31]. The papain-like
protease 1 (PL1pro) domain is present in nonstructural protein 3 (nsp3) of
alphacoronaviruses and subgroup 2a beta coronaviruses and the papain-like
protease 2 (PL2pro) is present in SARS-CoV.
In combination with the prior
characterization of PL2pro from other alpha
corona-viruses of human coronaviruses 229E, NL63, these viruses employ two
PLpros with overlapping specificities toward both viral and cellular substrates
[32]. The ZAP could regulate RNA virus degradation of SARS-CoV's and MERS-CoV's
RNA virus. Zn2+ ions are capable of inhibiting PLpro activity and
the zinc conjugates to inhibit SARS-CoV PLpro activity that targeting PLpro
with antiviral drug may have an advantage in not only inhibiting viral
replication but also inhibiting the dysregulation of signaling cascades
infected cells, leading to cell death [33]. Zn2+ inhibits
coronavirus and arterivirus RNA polymelase activity and zinc ionophores block
the virus replication that the combination of Zn2+ and pyrithione at
low concentrations inhibits the replication of SARS-CoV and arterivirus RNA [34].
High zinc ion concentration and
the addition of compounds that stimulate cellular import of zinc ions were
found to inhibit the replication of various RNA virus, influenza viruses,
respiratory syncytial virus and coronaviruses [34]. Further, zinc conjugated
complexes as SARS-CoV 3C-like protease inhibitors play important role for this
Zn2+-centered coordination pattern that the zinc-coordinating
inhibitor is tetrahedrally coordinated to the His40-Cys147 catalytic dyad of
CVB3 3Cpro [35,36]. ZAP' stress with antiviral activity and induced virus
replication are regulated upon virus infection to inhibit virus spread [37].
ZAP-70 kinase regulates HIV cell-to-cell spread that HIV usurps components of
the immunological synapse machinery to ensure its own spread through
cell-to-cell contacts [38]. An understanding of viral cell-to-cell transmission
spreading will enhance our ability to intervene in the efficient spreading of
viral infection [39].
Zinc-Binding
Domain
A novel Zinc-Binding
Domain (ZBD) is essential for formation of the functional Junin virus
envelope glycoprotein complex that the envelope glycoprotein of the Junin
arenavirus (GP-C) mediates entry into target cells through a pH-dependent
membrane fusion mechanism, in which this unusual motif may act to retain a
cleaved 58-amino-acid Stable
Signal Peptide (SSP) for its role in modulating membrane fusion activity [40].
Entry of the virus into the host cell is mediated by the viral envelope
glycoprotein, GPC that SSP was retained in GPC through interaction with a ZBD
in the cytoplasmic tail of transmembrane fusion of G2 subunits that Junin virus
ZBD displays a novel fold containing two zinc ions, in which the structural
basis for retention of the unique SSP submit suggests a mechanism whereby SSP
is positioned in the GPC complex to modulate pH-dependent membrane fusion [41].
Complex ZBD regulates replicative alterivirus helicase and controls mRNA decay
helicase [42]. Viral inhibitor p53 down-regulates SARS-CoV replications that
p53 inhibits replication of infectious SARS-CoV as well as of replicons and
human coronavirus NL63. Hence, HCoVs antagonize the viral inhibitor p53 via
stabilizing RCHY1 and promoting RCHY1-mediated p53 degradation [43].
Zinc-binding status having Zn2+ ions-centered coordination structure
could serve as the development of potential drugs for SARS therapies. A complex
zinc finger ZBD modulates the enzymatic activities of coronaviridae-Nidovirus
helicases, leading that the ZBD is critically involved in nidovirus replication
and transcription [44].
Enveloped viruses enter cells and initiate disease-causing cycles of replication that in all cases virus-cell fusion is executed by one or more viral surface glycoproteins denoted as the fusion protein, in which the structure and mechanisms on viral membrane fusion protein are important problems [45]. The membrane fusion reaction, membrane interaction, conformational changes of specialized virus envelope proteins and refolding reactions of specific fusion proteins can mediate both virus-cell fusion leading to infection and pathological cell-cell fusion, in which they are increasingly viewed as targets for antiviral intervention [45]. Thus, the virucidal activities of zinc-finger antiviral proteins for virus entry, replication and spread are represented in Table 1.
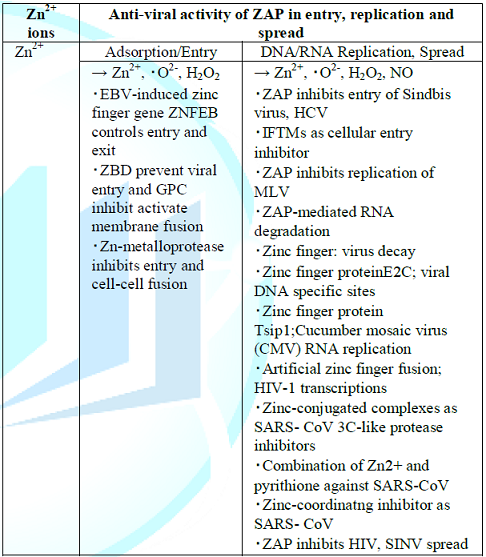
Accordingly,
anti-viral activities of ZNF, ZAP and ZBD are recognized by which Zn2+
ions bind RNA and facilitates viral RNA that is critical for RIG-1 like
receptor (RLR)-mediated innate immune response to RNA virus and highly diverse
fusion proteins have converged on the same overall strategy to mediate a common
pathway of membrane fusion, causing to lead enhancement of the anti-viral
activity.
Conclusion
The ZNFEB controls entry and exit
from cell cycling in activated lymphocytes. The designed polydactyl ZNF is
prepared consisting HIV-1 type integrase fused to the synthetic zinc finger
protein E2C. ZAP inhibits virus entry, replication and spread of certain
viruses and an understanding becomes necessary for ZAP-mediated viral RNA
degradation. ZAP inhibits the replication of certain viruses, regulates DNA and
RNA virus replication and promotes viral RNA degradation. The ZAP also inhibits
IAV protein expression, Retroviral RNA production and HIV-1 infection by
promoting the degradation of specific viral mRNAs. Further, the ZAP may
regulate RNA
virus degradations of HCoV, SARS-CoV's and MERS-CoV's RNA virus. HCoVs are
known as respiratory pathogens that HCoVs play only a minor role in causing
gastrointestinal illness in children year old.
The six known HCoVs have been
identified, namely HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1, SARS-CoV and
MERS-CoV. Zn2+ ions are capable of inhibiting PLpro activity and the
zinc conjugates to inhibit SARSCoV PLpro activity that targeting PLpro with
antiviral drug may have an advantage in not only inhibiting viral replication
but also inhibiting the dysregulation of signaling cascades infected cells,
leading to cell death. Zn2+ inhibits coronavirus and arterivirus RNA
polymelase activity and zinc ionophores block the virus replication. That the
combination of Zn2+ and pyrithione at low concentrations inhibits
the replication of SARS-CoV and arterivirus RNA. Zinc-conjugated complexes as
SARS-CoV 3C-like protease inhibitors play important role for this Zn2+-centered
coordination pattern that the zinc-coordinating inhibitor is tetrahedrally coordinated
to the His40-Cys147 catalytic dyad of CVB3 3Cpro.
ZAP's stress with antiviral
activity and induced virus replication are regulated upon virus infection to
inhibit virus spread. ZAP-70 kinase regulates HIV cell-to-cell spread that HIV
usurps the immunological components to ensure its own spread through
cell-to-cell contacts. A novel ZBD is essential for formation of the functional
Junin virus envelope glycoprotein complex. Entry of the virus into the host
cell is mediated by the viral envelope glycoprotein, GPC that SSP was retained
in GPC through interaction with a ZBD in the cytoplasmic tail of transmembrane
fusion of G2 subunits that Junin virus ZBD displays a novel fold containing two
zinc ions, in which the structural basis for retention of the unique SSP submit
suggests a mechanism whereby SSP is positioned in the GPC complex to modulate
pH-dependent membrane fusion.
Complex ZBD regulates replicative
arterivirus helicase and controls mRNA decay helicase. Thus, ZNF, ZAP and ZBD
specifically inhibit virus entry, replication and spread of many viruses. The
host-virus interaction, conformational changes of specialized virus envelope
proteins and refolding reactions of specific fusion proteins in an essential
steps entry, replication and spread of enveloped virus life cycle have been
worthy of remark in fascination that these diverse viral fusion protein could
be used in next-generation for therapeutic intervention in arenaviral disease.
Complex ZBD regulates replicative alterivirus helicase and controls mRNA decay
helicase. Viral inhibitor p53 down-regulates SARS-CoV replications that p53
inhibits replication of infectious SARS-CoV as well as of replicons and human
coronavirus NL63.
Hence, HCoVs antagonize the viral inhibitor p53 via stabilizing RCHY1 and promoting RCHY1-mediated p53 degradation. Enveloped viruses enter cells and initiate disease-causing cycles of replication that in all cases virus-cell fusion is executed by one or more viral surface glycoproteins denoted as the fusion protein, in which the structure and mechanisms on viral membrane fusion protein are important problems. Accordingly, virucidal activities of ZNF, ZAP and ZBD are recognized by which Zn2+ ions bind RNA and facilitate viral RNA that is critical for RLR-mediated innate immune response to RNA virus and highly diverse fusion proteins have converged on the same overall strategy to mediate a common pathway of membrane fusion, causing to lead enhancement of the anti-viral activity.
References
- Wessels I, Maywald M and Rink L. Zinc as gatekeeper of immune function (2017) Nutrients 9: 1-44.
- Ibs KH and Rink L. Immunity enhanced by trace elements (2003) J Nutr 133: 1452S-1456S.
- Read SA, Obeid S, Ahlenatiel C and Ahlenstiel G. The role of zinc in antiviral immunity (2019) Adv Nutr 10: 696-710. https://doi.org/10.1093/advances/nmz013
- Gammoh NZ and Rink L. Zinc in infection and inflammation (2017) Nutrients 9: 1-25.
- Dashti-Khavidaki S, Khalili H, Vahedi SM and Lessan-Pezeshki M. Serum zinc concentrations in patients on maintenance hemodialysis and its relationship with anemia, parathyroid hormone concentrations and pruritus severity (2019) Saudi J Kidney Dis Trans 21: 641-645.
- Ishida T. Review on the role of Zn2+ ions in viral pathogenesis and the effect of Zn2+ ions for host cell-virus growth inhibition (2019) American J Biomed Scie Res 2: 28-37. https://doi.org/10.34297/ajbsr.2019.02.000566
- Pium LM, Rink L and Haase H. The essential toxin: impact of zinc on human health (2010) Int J envir res public health 7: 1342-1365. https://doi.org/10.3390/ijerph7041342
- Lancellotti M, Pereira RFC, Cury GG and Hollanda LM. Pathogenic and opportunistic respiratory bacteria-induced apoptosis (2009) Brazilian J Infec Dis 13: 226-231. https://doi.org/10.1590/s1413-86702009000300014
- Navarro JM and Perez-Ruiz M. Antiviral immunity (2011) Curr Immuun Rev 7: 19-24. https://doi.org/10.2174/157339511794474244
- Jevsnik M, Steyer A, Zrim T, Pokorn M, Mrvič T, etal. Detection of human coronaviruses in simultaneously collected stool samples andnasopharyngeal swabs from hospitalized children with acute gastroenteritis (2013) Virol J 10: 1-7. https://doi.org/10.1186/1743-422x-10-46
- Bergantz L, Subra F, Deprez E, Delelis O and Richetta C. Interplay between intrinsic and innate immunity during HIV infection (2019) Cells 8: 922. https://doi.org/10.3390/cells8080922
- Martin-Vicente M, Medrano L, Resino S, García-Sastre A and Martínez I. TRIM25 in the regulation of the antiviral innate immunity (2017) Front immunol 8: 1-9. https://doi.org/10.3389/fimmu.2017.01187
- Rey FA and Lok SM. Common features of enveloped viruses and implications for immunogen design for next-generation vaccines (2018) Cell 172: 1319-1334. https://doi.org/10.1016/j.cell.2018.02.054
- Lee WYJ, Menhua R, Liang C and Sloan RD. IFITM proteins inhibit HIV-1 protein synthesis (2018) Sci Rep 8: 1-15. https://doi.org/10.1038/s41598-018-32785-5
- Crosse KM, Monson E, Beard MR and Helbig KJ. Interferon-simulated genes as enhancers of antiviral innate immunesignaling (2018) J Innate Immun 10: 85-93. https://doi.org/10.1159/000484258
- Tune CE, Pilon M, Saiki Y and Dosch HM. Sustained expression of the novel EBV-induced zinc finger gene, ZNFEB, is critical for the transition of B lymphocyte activation to oncogenic growth transformation (2002) J immunol 168: 680-688. https://doi.org/10.4049/jimmunol.168.2.680
- Tan W, Zhu K, Segal DJ, Barbas CF and Chow SA. Fusion protein consisting of HIV type 1 integrase and the designed polydactyl zinc finger protein E2C firect integration of viral DNA into specific sites (2004) J Virol 78: 1301-1313. https://doi.org/10.1128/jvi.78.3.1301-1313.2004
- Lian H, Zang R, Wei J, Ye W, Hu MM, et al. The zinc-finger protein ZCCHC3 binds RNA and facilitates viral RNA sensing and activation of the RIG-I-like receptors (2018) Immun 49: 438-448. https://doi.org/10.1016/j.immuni.2018.08.014
- Kim YS, Kim JM, Jung DL, Kang JE, Lee S, et al. Artificial zinc finger fusions targeting Sp1-binding sites and the trans-activator- responsive element potently repress transcription and replication of HIV-1 (2005) J Bio Chem 280: 21545-21552. https://doi.org/10.1074/jbc.m414136200
- Hur SU, Kim MJ, Kook B, Ham BK and Paek KH. A zinc finger protein Tsip1 controls cucumber mosaic virus infection by interacting with the replication complex on vacuolar membranes of the tobacco plant (2011) New Phytol 191: 746-762. https://doi.org/10.1111/j.1469-8137.2011.03717.x
- Wang X, Lv F and Gao G. Mutagenesis analysis of the zinc-finger antiviral protein (2010) Retrovirol 7: 1-9. https://doi.org/10.1186/1742-4690-7-19
- Schapiro JM, Libby SJ and Fang F. Inhibition of bacterial DNA replication by zinc mobilization during nitrosative stress (2003) PNAS 100: 8496-8501. https://doi.org/10.1073/pnas.1033133100
- Gao G, Guo X and Goff SP Inhibition of retroviral RNA production by ZAP, a CCCH-type zinc finger protein (2002) SCIE 297: 1703-1706.https://doi.org/10.1126/science.1074276
- Zhu V, Chen G, Lv F, Wang X, Ji X, et al. Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNA for degradation (2011) PNAS 108: 215834-215839. https://doi.org/10.1073/pnas.1101676108
- The Rockefeller University. Virus entry and virus-host interactions (2019) Lab Virol Infec Dis 15: 1.
- Bick MJ, Carroll JWN, Gao G, GoffS P, Rice CM, etal. Expression the zinc-finger antiviral protein inhibits alpha virus replication (2003) J Virol 77: 11555-11562. https://doi.org/10.1128/jvi.77.21.11555-11562.2003
- Tang Q, Wang X and Gao G. The short form of the zinc finger antiviral protein inhibits influenza A virus protein expression and is antagonized by the virus-encoded NS1 (2017) J Virol 91: 1-14. https://doi.org/10.1128/jvi.01909-16
- Villalon-Letelier F, Brooks A. Saunders P, Londrigan S and Reading P. Host cell restriction factors that limit influenza A infection (2017) Viruses 9: 376. https://doi.org/10.3390/v9120376
- Lim YX, Ng YL, Tam JP and Liu DX. Human coronaviruses:A review of virus-host interactions (2016) Diseases 4: 1-28. https://doi.org/10.3390/diseases4030026
- Ratia K, Singh K, Saikatendu KS, Santarsiero BD, Barretto N, et al. Severe acute respiratory syndrome coronavirus papain-like protease: Structure of a viral deubiquitinating enzyme (2006) PNAS 103: 58717-5722. https://doi.org/10.1073/pnas.0510851103
- Lee H, Lei H, Santarsiero BD, Gatuz JL, Cao S, et al. Inhibitor recognition specificity of MERS-CoV papain-like protease differs from that of SARS-CoV (2015) ACS Chem Biol 10: 1456-1465. https://doi.org/10.1021/cb500917m
- Wojdyla JA, Manolaridis I, Kasteren PB, Kikkert M, Snijder E, et al. Papain-like protease 1 from transmissible gastroenteritis virus: crystal structure and enzymatic activity toward viral and cellular substrates (2020) J Virol 84: 10063-10073. https://doi.org/10.1128/jvi.00898-10
- Baez-Santos YM, John SE and Mesecar AD. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds (2015) Antiviral Res 115: 21-38. https://doi.org/10.1016/j.antiviral.2014.12.015
- Velthuis JW, van den Worm SHE, Sims AC, Baric RS, Snijder EJ, et al. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture (2010) PLOS Patho 6: 1-10. https://doi.org/10.1371/journal.ppat.1001176
- Lee CC, Kuo CJ, Hsu MF, Liang PH, Fang JM, et al. Structural basis of mercury- and zinc-conjugated complexes as SARS-CoV 3C-like protease inhibitors (2007) FEBS Letters 581: 5454-5458. https://doi.org/10.1016/j.febslet.2007.10.048
- Lee CC, Kuo CJ, Ko TP, Hsu MF, Tsui YC, et al. Structural basis of inhibition specificities of 3C and 3C-like proteases by zinc-coordinating and peptidomimetic compounds (2009) J Bio Chem 284: 7646-7655. https://doi.org/10.1074/jbc.m807947200
- Law LMJ, Razooky BS, Li MMH, You S, Jurado A, et al. ZAP's stress granule localization is correlated with its antiviral activity and induced by virus replication (2019) PLOS Patho 15: 1-22. https://doi.org/10.1371/journal.ppat.1007798
- Sol-Foulon N, Sourisseau M, Porrot F, Thoulouze MI, Trouillet C, et al. ZAP-70 kinase regulates HIV cell-to-cell spread and virological synapse formation (2007) The EMBO J 26: 516-526. https://doi.org/10.1038/sj.emboj.7601509
- Mothes W, Sherer NM, Jin J and Zhong P. Virus cell-to-cell transmission (2010) J Virol 84: 8360-8368. https://doi.org/10.1128/jvi.00443-10
- York J and Nunberg H. A novel zinc-binding domain is essential for formation of the functional Junin-virus envelope glycoprotein complex (2007) J Virol 81: 13385-13391. https://doi.org/10.1128/jvi.01785-07
- Briknarova K, Thomas CJ, York J and Nunberg H. Structure of a zinc-binding domain in the junin virus envelope glycoprotein (2012) J Bio Chem 286: 1528-1536. https://doi.org/10.2210/pdb2l0z/pdb
- Deng Z, Lehmann KC, Li X, Feng C, Wang G, et al. Structural basis for the regulatory function of a complex zinc-binding domain in a replicative arterivirus helicase resembling a nonsense-mediated mRNA decay helicase (2014) Nucleic Acids Res 42: 3464-3477. https://doi.org/10.1093/nar/gkt1310
- Ma-Lauer Y, Carbajo-Lozoya J, Hein MY, Müller MA, Deng W, et al. p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLpro via E3 ubiquitin ligase RCHY1 (2016) PNAS 113: E5192-E5201. https://doi.org/10.1073/pnas.1603435113
- Seybert A, Posthuma CC, van Dinten LC, Snijder EJ, Gorbalenya AE, et al. A complex zinc finger controls the enzymatic activities of nidovirus helicases (2005) J Virol 79: 696-704. https://doi.org/10.1128/jvi.79.2.696-704.2005
- White JM, Deolos SE, Brecher M and Schornberg K. Structure and mechanisms of viral membrane fusion proteins: multiple variations on a common theme (2008) Crit Rev Biochem Mol Biol 44: 189-219. https://doi.org/10.1080/10409230802058320
Corresponding author
Tsuneo Ishida, Doctor of Science, under the retirement, 2-3-6, Saido, Midori-Ku, Saitama-Shi, Saitama-Ken, Japan, E-mail: ts-ishida@ac.auone-net.jp
Citation
Ishida
T. Virucidal
activities of zinc-finger antiviral proteins and zinc-binding domains for virus
entry, DNA/RNA replication and spread (2020) Edel J
Biomed Res Rev 2: 9-13.
Keywords
Zinc-finger antiviral protein, Virus entry, Replication
and spread, RNA degradation, SARS-CoV, PLpro, Zn2+ ion-coordination pattern


 PDF
PDF