Research Article :
Hau Tan Vo, Tha
Thi Nguyen and Vinh-Nghi Kim Ho At
the present time, polyphenolic compounds have attracted great interest due to
their roles in the prevention of degenerative diseases and have used as the
input material for manufacturing functional foods, nutraceutical and
pharmaceutical products. Previous researches have revealed the avocado seed is
rich source of polyphenolic compounds with antioxidant and antimicrobial
activities; which they could be used as a source of potent natural ingredients
and additives. In this study, extraction factors of polyphenols were optimized
for recovery yield by using response surface methodology and the obtained
polyphenol rich solution was encapsulated with different coating agents (Maltodextrin-MD
and Gum Arabic-GA) as well as their mixtures. A Box-behnken design was used to
investigate the effects of three independent variables including ethanol
concentration (X1:35-65%, v/v), solvent to solid ratio (X2:8-12,
v/w) and extraction time (X3:1.0-3.0 h). The result shown that the
optimized extraction conditions were using ethanol concentration of 40% (v/v),
ratio of solvent to solid at 12:1 and extraction time of 1.5 h. Under the
conditions, the experimental recovery yield of polyphenols is 83.1% which is
well matched with the predicted yield of 82.5%. Micro particle prepared by
20:80 of MD:GA ratio as coating agent can be selected for encapsulation of the
polyphenolic compounds. Avocado
(Persea
americana Mill.) is an evergreen
tree native to Central America that now widely cultivated in the tropical and
subtropical regions of the world for edible fruits, which are very rich in oil
[1]. In 2017, world avocado production was approximately 5.92 million metric tons.
This tree was first introduced into the Lam Dong province of Vietnam in 1940 by
the French [2,3]. Although no statistical figures are available on the area and
output of avocado, the tree is widely grown in the uplands of Vietnam such as
Dong Nai, Ba Ria-Vung Tau, Lam Dong, Dak Lak and Phu Tho with various local
names given to them according to their fruit shape and quality. The
fruit of the plant, also called avocado (or avocado pear or alligator pear), is
commercially importance. The edible part of the fruit is rich in unsaturated
fatty acids, vitamins B, C and E, and other nutrients [4]. The avocados are
mainly consumed as a fresh fruit but at the moment many value added products
have manufactured as guacamole, avocado pulp and avocado oil [5]. Industrial
processing of avocados generates a large amount of peels and seeds as waste
which can cause environmental problems. The avocado seed contents up 16% of the
total weight of the fruit and has a long history of ethno botanical use [6].
They are a rich source in phytochemicals, especially polyphenolic compounds
such as hydroxycinnamic acid derivatives, flavonoids
and proanthocyanidins with many bioactivities such as anticancer, antidiabetic
effect antihypertensive effect [7-10] and antioxidant and antibacterial
properties [11-16]. Polyphenols
are a group of plant-derived secondary metabolites with phenolic structural
features. They can be divided into at least 10 different classes depending on
their basic chemical structure of aglycones
such as phenolic acid derivatives, flavonoids and proanthocyanidins [17].
Currently, the compounds have attracted great interest due to their roles in
the prevention of degenerative diseases, particularly cancers, cardiovascular
diseases and neurodegenerative diseases [18]. They have used as the input
materials for manufacturing functional foods, nutraceutical and pharmaceutical
products. However, the effectiveness of polyphenols depends on preserving the
stability, bioactivity and bioavailability of the active ingredients. Moreover,
the unpleasant taste of most polyphenolic compounds also limits their
application. Therefore, encapsulation process can be a useful method to alleviate
these deficiencies. Encapsulation
may be defined as a process to entrap substances (active agents) within another
substance (wall materials) [19]. Up to now, many encapsulation technologies of
polyphenols have been used effectively such as spray drying, liposome
entrapment and emulsion. In the food industry, the encapsulation
process is a useful tool to improve delivery of bioactive molecules into
foods [20]. Besides, the process can be applied for protecting the core
material from degradation by the surrounding environment, preventing unwanted
flavor or taste of core material as well as modifying the nature of the
original material for easier handling [21]. Nowadays,
there is a growing interest in finding phytochemicals as alternatives to the
synthetic substances that are commonly used in the food, pharmaceutical and
cosmetic industries. The research results about polyphenol rich extract of
avocado seed have revealed their potential usage as they contribute to enhance
the nutritional and technological value of the meat products through their
antioxidant action [22-23]. Response
Surface Methodology (RSM) consists of a group of mathematical and
statistical techniques that are based on the fit of empirical models to the
experimental data obtained in relation to experimental design [24]. It is the
one of most effective tools for optimizing the process when many factors and
interactions affect the desired response. RSM usually uses an experimental
design such as Box-behnken or central composite to fit a second order
polynomial [25-26]. The
purpose of this study was ·
To
optimize the extraction parameters of polyphenols from the avocado seeds using
RSM ·
To
study effect of encapsulating agents on physiochemical characteristics of
polyphenol rich micro particles. Materials Avocado
fruits (named “Bơ Sáp” in Vietnamese) were purchased from the local market in
Ho Chi Minh City, Vietnam and kept at room temperature until they reached
ready-to-eat ripeness. MD (DE=10-13) and GA supplied by Sigma-Aldrich Chemical
Co. were used as coating materials. Other chemicals either HPLC reagent grades
or the highest purity were available. Preparation of Avocado Seed Powder (ASP) Avocado
seeds were manually separated from the flesh and cleaned. The seeds were sliced
with the average thickness of the sliced seed samples about 2.5 mm then they
were soaked in a 1.5% w/v citric acid solution for 20 minute (the citric acid
used as an inhibitor of enzymatic browning). The pretreated slices were dried
in a hot air oven at 70oC for 3h. The
dried slices with moisture of 7.04% were ground into powder using Grindomix
machine (Retsch, GM 200, Germany) and stored at -25oC until use. Extraction Procedure Solid-liquid
extractions were carried out at temperature 60oC.
One hundred grams of ASP sample (126.02 ± 0.1 mg GAE/g) were blended with
ethanol solvent at concentrations (35-65%, v/v), ratios of solvent to solid
(8-12 v/w) and extraction time (1.0-3.0 h) as specified by the experimental
design (Table 1). After the
extraction, liquid extracts were separated from solids by filtration and
removed ethanol in a rotary evaporator at 55oC. The recovery yield
of polyphenols (RYP) from ASP was calculated according to equation 1. Preparation of the Polyphenol Rich Micro
particles MD
and GA mixed with different ratios of 10:0, 8:2, 6:4, 4:6, 2:8 and 0:10 were
used as coating materials. Polyphenolic
extract after removing solid and ethanol (312.5 ml, total polyphenolic
content=21 mg GAE/ml and dry matter content=2.4 g/100 ml) was previously heated
at 45oC with constant stirring. 42.5 g MD:GA
mixture with above ratios was added to form homogeneous solutions. Total solid
content of the solutions before spray drying was corrected to 10 by adding to
137.5 ml distilled water. The obtained solutions were fed to a mini spray dryer
(B-290, Büchi). The spray dryer was operated at inlet temperature 160 ± 2oC.
The air flow and rate of feeding were 550L h-1 and 10 ml min-1,
respectively. The powders obtained were kept in dark container at -20oC
until analysis. Total Polyphenol Content (TPC) Determination The
Total Polyphenol Content of each extract was determined by the Folin-Ciocalteu
assay with minor modifications [8]. The extracts (1 ml) were mixed with 5 ml of
1:10 diluted Folin-Ciocalteus
phenol reagent, followed by 4 ml of sodium carbonate (7.7%, w/v) and
allowed to stand for 30 min in the dark at room temperature then the absorbance
was read at 760 nm using a spectrophotometer (Shimadzu, Japan). The polyphenol
content was calculated as mg of Gallic Acid Equivalents (GAE) per gram of dry
matter from a standard curve of Gallic acid. To
extract polyphenols for TPC determination from ASP, 0.2g of samples were
extracted in 10 ml acetone/water (70:30, v/v) for 30 min [15]. After the
extraction, the extract was centrifuged at 3000 rpm for 15 min. The supernatant
was collected and the residue was re-extracted once more. The two supernatants
were combined and dried by using a rotary evaporator at 55oC.
The residue was dissolved in 10ml of distilled water and kept in dark container
at 5oC until analysis. For
determination of TPCm and Surface Polyphenol Content (SPCm)
of micro particles, capsules of 1 g were dissolved in 10ml of methanol: acetic
acid: water (50:8:42,v/v/v) or 10ml ethanol: methanol (50:50, v/v),
respectively. The supernatants were centrifuged at 3500rpm for 15min and then
filtered [27]. TPCm and SPCm were quantified as described
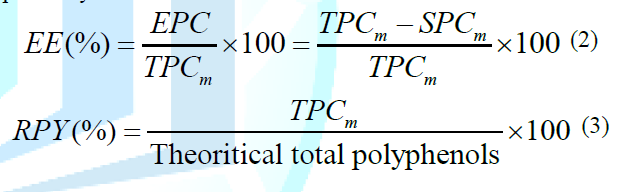
above. The Encapsulation
Efficiency (EE) and the Recovered Polyphenol Yield (RPY) from spray dried
experiments were calculated according to equations 2-3, respectively. Where
EPC was the encapsulated polyphenol content which was calculated by subtracting
total polyphenol content (TPCm) of micro particles from surface
polyphenol content (SPCm) of micro particles. Moisture Content and Water Solubility
Index The
moisture content of micro particles was determined based on the loss in weight
between samples before and after drying at 105 ± 2oC. In
order to evaluate the solubility of the micro particles, the Water
Solubility Index (WSI) was determined using by Anderson method with minor
modifications [28]. One gram of powder samples was added to 12 ml distilled
water, mixed and incubated in a water bath at 30oC
for 30 min. After incubation, samples were centrifuged at 3500 rpm for 15 min.
The supernatants were collected and evaporated at 105 ± 2oC
until obtaining a constant weight. WSI was expressed as in equation 4. Experimental Design and Statistical
Analysis In
this study, RSM was used to predict the optimum extraction conditions of
polyphenol compounds from ASP by using Design-Expert software (version 9.0,
Stat-Ease Inc., Minneapolis, MN, USA). The Box-Behnken
Design (BBD) with a quadratic model was selected to investigate the
combined effects of three independent variables while extraction temperature was
fixed at constant rate of 60oC (determinates
after several preliminary experiments, data not shown). The independent
variables were ethanol concentration (35-65%, v/v), ratio of the solvent to
solid (8-12, v/w) and extraction time (1.0-3.0 h). Experimental design scheme
derived from Design-Expert and response value (Y, recovery yield of
polyphenols) were presented in Table 1. The actual values were coded at three
levels: −1, 0, and +1 according to the following equation: Where
Xi is the coded value, xi is the corresponding actual
value, x0 is the actual value in the center of the domain, and Δx is
the increment of xi corresponding to a variation of 1 unit of X. Experimental
data were fitted to a quadratic polynomial model and regression coefficients obtained.
The computer-generated
quadratic model used in the response surface was as follows: Where
Y denotes the dependent variable. The coefficients of the polynomial equation
were represented by β0 (intercept), βi (linear effects),
βii (quadratic effects), and βij (cross product effects).
Xi represented the coded levels of independent variables. The terms
XiXj and Xi2 were expressed as the
interaction and quadratic terms, respectively. Design-Expert
software was used to estimate the response of each set of experimental design
and optimized conditions. The quality of the fitted model was expressed by the
coefficient of determination R2, the adjusted determination
coefficient R2adj as well as the predicted determination
coefficient R2pred and statistical significance of the model
was determined by F-test. All
treatments were done in triplicate and the results were expressed as a mean (±
SD) for each treatment. The significant difference between treatments reported
at p ≤ 0.05. Extraction optimization Model fitting: The study used
RSM to develop a prediction model for optimizing conditions of polyphenol
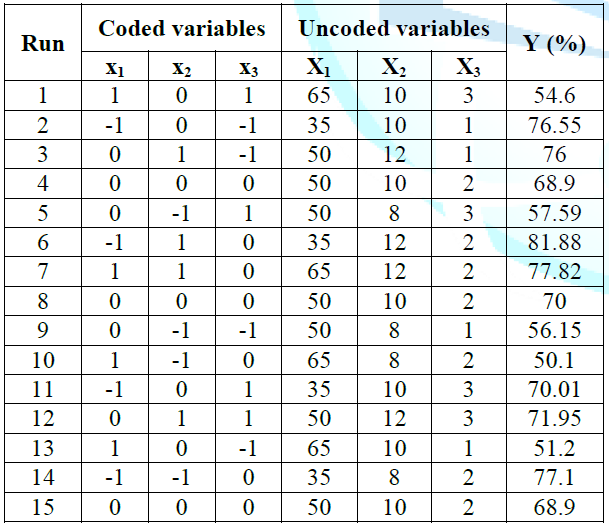
extraction from ASP. The experimental conditions and experimental data of 15 runs
containing 3 replicates at center point were presented in Table 1.
Experimentally obtained values for polyphenol
recovery varied from 50.10% to 81.88% and the highest recovery was at the
point with 35% of ethanol concentration, the solvent to solid ratio of 12 and
120 min of extraction time. By performing multiple regression analysis on the
experimental data, the model for the response variable (recovery yield of
polyphenols from ASP) could be expressed in form of coded values by the
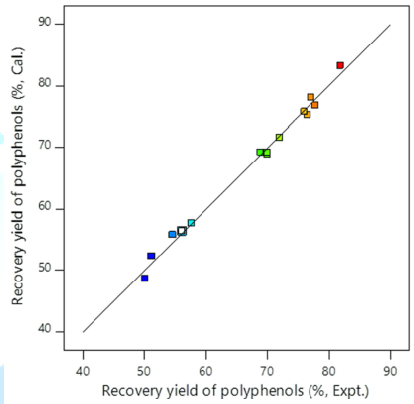
quadratic polynomial equation as follows: The plot of experimental values of the recovery yield of polyphenols versus those calculated from equation (7) indicated a good fit, as shown in Figure 1. To
test the significance and adequacy of the model, the Analysis
of Variance (ANOVA) for the quadratic model was performed. The lack of fit
test measures the failure of the model to represent the data in the
experimental domain at points which are not included in the regression, which determines
whether the selected model is adequate to explain the experimental data or
another model should be reselected [29]. As shown in Table 2, the lack of fit
test was not significant relative to the pure error (p>0.05) and implied
that the model equation was available. The model F-value of 66.1 (p ~ 0.0001)
revealed the model was significant. There was only a 0.01% chance that and this
large F-value could occur due to noise. The
results of the analysis of variance also generated the determination
coefficients for the model as shown in Table 2. For the fitted model, the
coefficient of determination (R2), which is a measure of degree of
fit, was 0.9917. This indicated that only 0.83% of the total variations were
not explained by the fitted model as well as revealed the satisfactory
correlation between actual values and predicted ones. Lundstedt, et al.
suggested that, for a good fit of a model, R2 should be at least
0.80 [30]. Moreover,
the adjusted determination coefficient (R2adj, 0.9767)
was high and very close to R2. The higher the value of R2adj
is, the deeper the correlation between the observed and predicted values
performs [31]. R2prep (0.9738) was in reasonable
agreement with R2adj. CV (coefficient
of variation), which indicates the degree of precision with that the
experiments are compared, was 2.39. A relatively low value of CV disclosed a
better precision and reliability of quadratic polynomial model adequate
precision compares the range of the predicted values at the design points to
the average prediction error and a ratio greater than 4 is desirable. The value
of adequate precision was 26.3372 as shown in Table 2. Therefore, the model is adequate for prediction in the
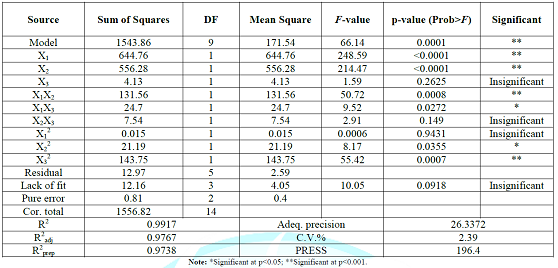
range of experimental variables and could be used to navigate the design space. Table 2: The analysis of
variance (ANOVA) table for response surface quadratic model. Effects of
Extraction Conditions on Recovery Yield of Polyphenols: The effects of
extraction conditions of ASP on polyphenol recovery yield by the regression
coefficients of fitted second-order polynomial are presented in Table 2 and the
significance of each coefficient was determined using F-value and p-value. It could be seen
that the effects of ethanol concentration and the solvent to solid ratio (X1,
and X2; p<0.05) were the major contributing factors to the
recovery yield of polyphenols, while extraction time had no significant effect
(X3; p>0.05) within the experimental range. In addition, it was
evident that coefficients (X1X2, X1X3,
X22, and X32) were significant at
the level of p<0.05, whereas the other coefficients were insignificant (X1X3,
X12; p>0.05). To
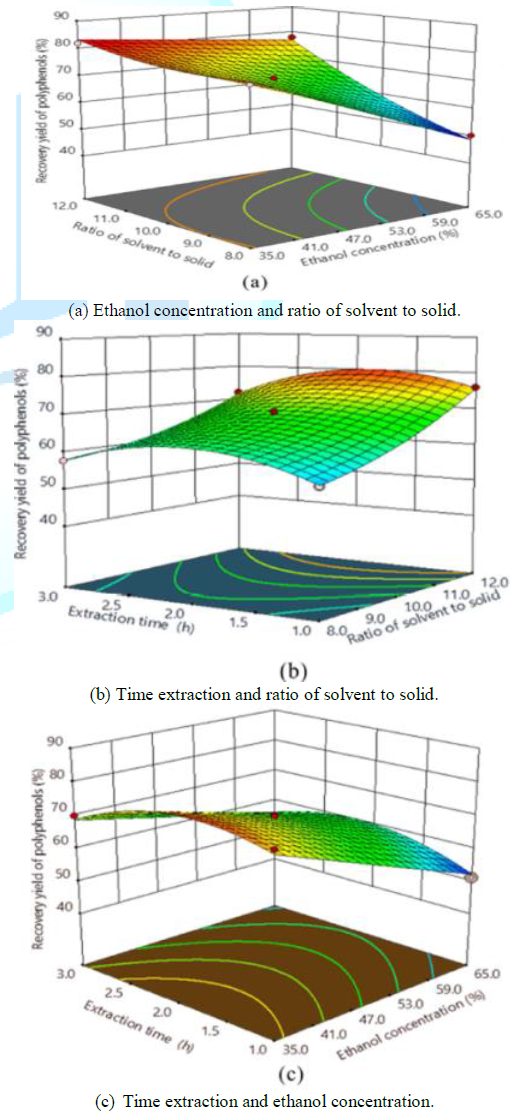
aid visualization, the relationship between independent variables and response
variable of polyphenolic extraction from ASP was graphically represented by 3D
response surfaces generated by the model as in Figure 2 and the relationships could be intuitively conveyed as two
variables were depicted in same plots while the other variable was kept at
level 0. The interactions of ethanol concentration (X1) with solvent
to solid ratio (X2) and the extraction time (X3) on the
recovery yield of polyphenols shown in Figures 2(a) and 2(b), respectively. The
yield of polyphenols increased rapidly with the increment of solvent to solid
ratio and reduction of ethanol concentration. The results demonstrated that the
interaction between ethanol concentration (X1) and solvent to solid
ratio (X2) was very remarkable but this interaction was against each
other. The yield also increased lightly along with the increment of extraction
time and diminution of ethanol concentration, while it declined lightly with
higher extraction time after a critical value of 2 h. Figure
(2c) suggested that the interaction between solvent to solid ratio (X2)
and time extraction (X3) was not significant. Curvature of the
response surface in this Figure may be due to quadratic effects of solvent to
solid ratio (X2) and time extraction (X3) on the
response. Determination of
Optimum Conditions and Model Verification: From the model, the optimum
conditions for polyphenolic extraction from ASP were obtained by using Design-Expert
software was presented as in Table 3.
Under optimum conditions, recovered yield of 82.5% polyphenols was predicted.
The suitability of the model equation for predicting the optimum response value
was tested by additional independent experiments (triplicate) using the
recommended optimum conditions in Table 3. The result has shown the
experimental recovery yield of polyphenols (83.1%) was not significantly
difference from the predicted value (82.5%). Physicochemical Properties of Micro
Particles The
polyphenol rich extract from ASP at optimum conditions as in Table 3 was used
for preparing spray dried solution with different mixtures of MD and GA as
coating agents. After spray
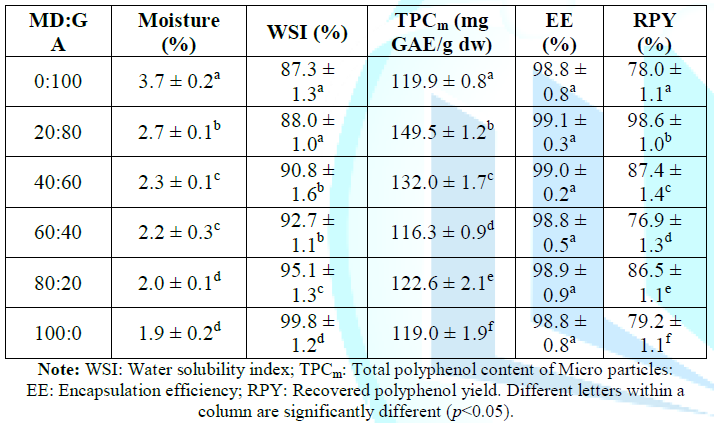
drying, the results of physiochemical evaluation are shown as in Table 4. As
can be seen in Table 4, the moisture
content of the micro particles ranges from 1.9% to 3.7% which decreases with
the increment of the MD fraction, reaching the lowest value when only MD (100%)
was used as coating agent, while the decreasing of the GA fraction rises WSI,
from 87.3% to 99.8% when MD:GA ratio ranges from 0:100 to 100:0 due to
solubility of GA in water is lower than that of MD. Although EE was almost
unchanged at any MD:GA ratio but the total polyphenol content in the micro
particle at 20:80 of MD:GA ratio had the highest value (149.5 mg GAE/g). RPY at
all experimental points varies from 78.0% to 98.6%. The
exploitation of waste from fruit and vegetable processing as a source of
bioactive compounds is a promising field and it offers a new avenue for
industrial growth and waste management. Extraction is the first and the most
important step in the recovery and purification of bioactive compounds from
plant materials and it was significantly influenced by many process factors
such as solvent, temperature and solvent to solid ratio [32-34]. Therefore,
optimizing the extraction
process in order to improve recovery of added-value compounds represents a
necessary technological innovation for the benefit of related industries. Despite
several disadvantages such low recovery yield and use of high solvent volumes
but solvent extraction techniques have been mostly used for the recovery of
polyphenolic compounds from plant materials due to their simple operation, wide
range of applicability and low outlay [35]. Many solvents can be selected to
extract these compounds such as ethyl acetate, acetone, propanol, ethanol, methanol
and water but the selection of ethanol and water as extraction solvent
throughout the study because they are safer for human consumption and less
toxic as compared to other organic solvents [36]. Moreover,
binary solvent system was found superior to the mono-solvent system due to the
compositions as well as the structure and physicochemical properties of
polyphenolic compounds from different plant sources [37]. For food and
pharmaceutical industries, target compound recovery from plant materials by
extraction is very significant when higher yield means lower production cost.
In this study, the results obtained from analysis of experimental model have
indicated that the effects of ethanol concentration and the solvent to solid
ratio were the major contributing factors to the recovery yield of polyphenol
from ASP as former reports and their effects depend on the polyphenol
composition of the plant materials using for extraction [38-42]. Shi, et al.
reported the polyphenol content extracted from grape seed increased when
ethanol concentration decreased and the best the ethanol concentration obtained
at 50% [43]. Polyphenol extraction was highly dependent on the solvent to solid
ratio reported by Pompeu, et al and the ratio was at 40:1 (v/w) in extraction
polyphenols from Euterpe
oleracea fruits [44]. Our
optimized extraction conditions were similar with Boyadzhieva, et al. wherein
their conditions obtained by using one factor at a time experiments with results
ethanol concentration of 30% (v/v), ratio of solvent to solid at 8 and
extraction time of 60 min from the avocado seed material but the polyphenol recovery
yield has not reported [45]. The recovery yield of polyphenols increased when
ethanol concentration was decreased due to polyphenolic composition of ASP
containing lots of polarity compounds such as procyanidins.
Moreover, water increases the contact surface area between plant matrix and
solvent, increasing the swelling capability of plant material which results in
increasing extraction efficiency. Increase in the recovery yield of polyphenols
under higher solvent to solid ratio is based on the mass transfer principles
where the driving force for mass transfer is considered to be the concentration
gradient between the solid and the solvent. At a lower ratio, the solvent can
attain saturation state soon during extraction. Polyphenolic
compounds originate from plant have recently obtained a great attention due to
their bioactive roles. However, they are sensitive and they can be easily
affected by physicochemical factors that create a great challenge to
incorporate them into the food products [19]. Hence, the encapsulation process
becomes an effective strategy to overcome this problem. There are many
different encapsulation methods for bioactive compounds but spray drying is an
industrial and economical method which is commonly used to transform the liquid
products into dry powders [46]. In this method, the sensitive compounds are
covered within the carrier material, which leads to their protection against
environmental disadvantages. Previous studies have revealed that the type and
characterization of carrier materials influence on many properties of
encapsulated micro particles [47-50]. Therefore, choosing the correct wall
material is the important step to produce efficient encapsulated powders. MD
and GA have been frequently used as carrier materials for encapsulation of
plant polyphenols due to their high solubility, good biocompatibility, optimum
viscosity and safety. Our results have shown that physicochemical
properties of micro particles were influenced by the carrier type as well as
their mixture ratio, which were similar to previous reports [51-52]. RPY and EE
are the most important indicators which shows the efficiency of the spray dried
process and they gained the best values at 20:80 of MD:GA ratio in this study (Table
4). Micro particles produced with GA presented higher moisture contents in
compared with MD. This may be due to higher water holding capacity of GA than
MD. This similar behavior also observed by Akhavan Mahdavi, et al. when
studying microencapsulation of natural anthocyanins from barberry fruits [53].
Solubility of micro particles (evaluated by WSI) is an important physiochemical
property that influences functional characteristics of micro particles in food
system. Our data shown that high solubility of all samples has been noticed. Avocado
seeds can be used as a raw material to extract bioactive polyphenols. The RSM
based on the BBD was successfully used to optimize process parameters for
polyphenolic extraction from ASP. The optimum conditions in the polyphenolic
extraction were using ethanol concentration of 40% (v/v), solid to solvent
ratio at 1:12 and extraction time of 1.5 h. Under the conditions, the
experimental yield of polyphenols is 83.1%. Besides, the characteristics of
polyphenol rich micro particles were also determined. Micro particle prepared
by 20:80 of MD:GA ratio as coating agent can be selected for encapsulation of
polyphenolic compounds from ASP. 1.
Giffoni
J, Salles E, Aguiar R, Nogueira R, Costa J, et al. Chemical composition,
toxicity and larvicidal and antifungal activities of Persea americana (avocado) seed extracts (2009) Rev Soc Bras Med
Trop 42: 110-113. http://dx.doi.org/10.1590/S0037-86822009000200003 2.
http://www.fao.org/faostat/en/#data/QC
Accessed 19/11/2019. 4.
Dreher
ML and Davenport AJ. Hass avocado composition and potential health effects
(2013) Crit Rev Food Sci Nutr 53: 738-750. http://dx.doi.org/10.1080/10408398.2011.556759 5.
Duarte
PF, Chaves MA, Borges CD and Mendonca CRB. Avocado: characteristics, health
benefits and uses (2016) Cienc Rural 46: 747-754. http://dx.doi.org/10.1590/0103-8478cr20141516
6.
Dabas
D, Shegog RM, Ziegler GR and Lambert JD. Avocado (Persea americana) seed as a
source of bioactive phytochemicals (2013) Curr Pharm Design 19: 6133-6140. http://dx.doi.org/10.2174/1381612811319340007 7.
Rosero
CJ, Cruz S, Osorio C and Hurtado N. Analysis of phenolic composition of
byproducts (seeds and peels) of avocado (Persea
americana Mill.) cultivated in Colombia (2019) Molecules 24: 3209-3226. http://dx.doi.org/10.3390/molecules24173209 8.
Lee
SG, Yu MH, Lee SP and Lee IS. Antioxidant activities and induction of apoptosis
by methanol extracts from avocado (2008) J Korean Soc Food Sci Nutr 37:
269-275. https://doi.org/10.3746/jkfn.2008.37.3.269
9.
Edem
DO. Hypoglycemic effects of ethanolic extracts of alligator pear seed (Persea americana Mill.) in rats (2009)
European J Sci Res 33: 669-678. 10.
Kate
IE and Lucky OO. Biochemical evaluation of the ethnomedicinal uses of the seeds
of Persea americana Mill. (Family
Lauraceae) (2009) World J Med Sci 4: 146-146. 11.
Wang
W, Bostic TR and Gu L. Antioxidant capacities, procyanidins and pigments in
avocados of different strains and cultivars (2010) Food Chem 122: 1193-1198. https://doi.org/10.1016/j.foodchem.2010.03.114
12.
Kosíska
A, Karamać M, Estrella I, Hernández T, Bartolomé B, et al. Phenolic compound
profiles and antioxidant capacity of Persea
americana Mill. Peels and seeds of two varieties (2012) J Agric Food Chem
60: 4613-4619. https://doi.org/10.1021/jf300090p 13.
Segovia
FJ, Hidalgo GI, Villasante J, Ramis X and Almajano MP. Avocado Seed: A
Comparative Study of Antioxidant Content and Capacity in Protecting Oil Models
from Oxidation (2018) Molecules 23: 2421-2434. https://doi.org/10.3390/molecules23102421
14.
Widsten
P, Cruz CD, Fletcher GC, Pajak MA and McGhie TK. Tannins and extracts of fruit
byproducts: Antibacterial activity against foodborne bacteria and antioxidant
capacity (2014) J Agric Food Chem 62: 11146-11156. https://doi.org/10.1021/jf503819t 15.
Rodríguez-Carpena
JG, Morcuende D, Andrade MJ, Kylli P and Estévez M. Avocado (Persea americana Mill.) phenolics, in
vitro antioxidant and antimicrobial activities, and inhibition of lipid and
protein oxidation in porcine patties (2011) J Agric Food Chem 59: 5625-5635. https://doi.org/10.1021/jf1048832 16.
Araújo
RG, Rodriguez-Jasso RM, Ruiz HA, Pintado MME and Aguilar CN. Avocado
by-products: nutritional and functional properties (2018) Trends Food Sci
Technol 80: 51-60. https://doi.org/10.1016/j.tifs.2018.07.027
17.
Martin
KR and Appel CL. Polyphenols as dietary supplements: A double-edged sword
(2010) Nutr Diet Suppl 2: 1-12. https://doi.org/10.2147/NDS.S6422
18.
Tsao
R. Chemistry and biochemistry of dietary polyphenols (2010) Nutrients 2:
1231-1246. 19.
Fang
Z and Bhandari B. Encapsulation of polyphenols- a review (2010) Trends Food Sci
Technol 21: 510-523. https://doi.org/10.1016/j.tifs.2010.08.003 20.
Jeyakumari
A, Zynudbeen AA and Parvathy U. Microencapsulation of bioactive food
ingredients and controlled release-a review (2016) MOJFPT 2: 00059-00067. https://doi.org/10.15406/mojfpt.2016.02.00059 21.
Desai
KDH and Park HJ. Recent developments in microencapsulation of food ingredients
(2005) Dry Technol 23: 1361-1394. https://doi.org/10.1081/DRT-200063478
22.
Utrera
M, Rodríguez-Carpena JG, Morcuende D and Estévez M. Formation of lysine-derived
oxidation products and loss of tryptophan during processing of porcine patties
with added avocado byproducts (2011) J Agric Food Chem 60: 3917-3926. https://doi.org/10.1021/jf3001313 23.
Rodríguez-Carpena
JG, Morcuende D and Estévez M. Avocado by-products as inhibitors of color
deterioration and lipid and protein oxidation in raw porcine patties subjected
to chilled storage (2011) Meat Sci 89: 166-173. https://doi.org/10.1016/j.meatsci.2011.04.013
24.
Bezerra
MA, Santelli RE, Oliveira EP, Villar LS and Escaleira LA. Response surface
methodology (RSM) as a tool for optimization in analytical chemistry (2008)
Talanta 76: 964-977. https://doi.org/10.1016/j.talanta.2008.05.019
25.
Ferreira
SLC, Bruns RE, Ferreira HS, Matos GD, David JM, et al. Box-Behnken design: an
alternative for the optimization of analytical methods (2007) Anal Chim Acta
597: 179-186. https://doi.org/10.1016/j.aca.2007.07.011
26.
Bas
D and Boyaci IH. Modeling and optimization I: Usability of response resurfaces
methodology (2007) J Food Eng 78: 836-845. https://doi.org/10.1016/j.jfoodeng.2005.11.024
27.
Saénz
C, Tapia S, Chávez J and Robert P. Microencapsulation by spray drying of
bioactive compounds from cactus pear (Opuntia ficus-indica) (2009) Food Chem
114: 616-622. https://doi.org/10.1016/j.foodchem.2008.09.095
28.
Anderson
RA. Water absorption and solubility and amylograph characteristics of
roll-cooked small grain products (1982) Cereal Chem 59: 265-269. 29.
Zhong
K and Wang Q. Optimization of ultrasonic extraction of polysaccharides from
dried longan pulp using response surface methodology (2010) Carbohydr Polym 80:
19-25. https://doi.org/10.1016/j.carbpol.2009.10.066
30.
Lundstedt
T, Seifert E, Abramo L, Nyström BTA, Pettersen J and Bergman R. Experimental design
and optimization (1998) Chemometr Intell Lab 42: 3-40. https://doi.org/10.1016/s0169-7439(98)00065-3
31.
Ravilumar
K, Ramalingam S, Krishnan S and Balu K. Application of response surface
methodology to optimize the process variables for reactive red and acid brown
dye removal using a novel adsorbent (2006) Dyes and Pigm 70: 18-26. https://doi.org/10.1016/j.dyepig.2005.02.004
32.
Mota
M, Pinto PCR, Novo G, Sousa G, Guerreiro O, et al. Extraction of polyphenolic
compounds from Eucalyptus globulus bark: process optimization and screening for
biological activity (2012) Ind Eng Chem Res 51: 6991-7000. https://doi.org/10.1021/ie300103z 33.
Çam
M and İçyer NC. Phenolics of pomegranate peels: extraction optimization by
central composite design and alpha glucosidase inhibition potentials (2015) J
Food Technol 52: 1489-1497. https://doi.org/10.1007/s13197-013-1148-y
34.
Chan
SW, Lee CY, Yap CF, Mustapha WAW and Ho CW. Optimisation of extraction
conditions for phenolic compounds from limau purut (Citrus hystrix) peels
(2009) Int Food Res J 16: 203-213. 35.
Brglez Mojzer E, Knez Hrnčič M, Škerget M, Knez Ž
and Bren U. Polyphenols: extraction methods, antioxidative action,
bioavailability and anticarcinogenic effects (2016) Molecules 21: 1-38. https://doi.org/10.3390/molecules21070901 36.
Spigno
G, Tramelli L and De Faveri DM. Effects of extraction time, temperature and
solvent on concentration and antioxidant activity of grape marc phenolics
(2007) J Food Eng 81: 200-208. https://doi.org/10.1016/j.jfoodeng.2006.10.021
37.
Thoo
YY, Ho SK, Liang JY, Ho CW and Tan CP. Effects of binary solvent extraction
system, extraction time and extraction temperature on phenolic antioxidants and
antioxidant capacity from mengkudu (Morinda citrifolia) (2010) Food Chem 120:
290-295. https://doi.org/10.1016/j.foodchem.2009.09.064
38.
Uysal
S, Cvetanović A, Zengin G, Đurović S and Aktumsek A. Optimization of the
extraction process of antioxidants from loquat leaves using response surface
methodology (2017) J Food Process Preserv 41: 1-8. https://doi.org/10.1111/jfpp.13185
39.
Chu
B, Shi Y, Li Z, Tian H, Li W and Wang Y. Optimization of gentisides extraction
from Gentiana rigescens Franch ex Hemsl by response surface methodology (2015)
J Anal Methods Chem 2015: 1-8. https://doi.org/10.1016/j.foodchem.2015.08.008
40.
Ćujić
N, Šavikin K, Janković T, Pljevljakušić D, Zdunić G and Ibrić S. Optimization
of polyphenols extraction from dried chokeberry using maceration as traditional
technique (2016) Food Chem 194: 135-142. https://doi.org/10.1016/j.foodchem.2015.08.008
41.
Singh
A, Kuila A, Yadav G and Banerjee R. Process optimization for the extraction of
polyphenols from okara (2011) Food Technol Biotechnol 49: 322-328. 42.
Prasad
KN, Kong KW, Ramanan RN, Azlan A and Ismail A. Selection of experimental domain
using two-level factorial design to determine extract yield, antioxidant
capacity, phenolics, and flavonoids from Mangifera pajang Kosterm (2012) Sep
Sci Technol 47: 2417-2423. 43.
Shi
J, Yu J, Pohorly J, Young JC, Bryan M and Wu Y. Optimization of the extraction
of polyphenols from grape seed meal by aqueous ethanol solution (2003) JFAE 1:
42-47. 44.
Pompeu
DR, Silva EM and Rogez H. Optimisation of the solvent extraction of phenolic
antioxidants from fruits of Euterpe oleracea using response surface methodology
(2009) Biores Technol 100: 6076-6082. https://doi.org/10.1016/j.biortech.2009.03.083
45.
Boyadzhieva
SS, Georgieva SS and Angelov G. Optimization of the extraction of natural
antioxidants from avocado seeds (2018) Bulg Chem Commun 50: 80-84. 46.
Orsat
V and Murugesan R. Spray drying for the production of nutraceutical
ingredients-A review (2012) Food Bioprocess Technol 5: 3-14. https://doi.org/10.1007/s11947-011-0638-z
47.
Boonchu
T and Utama-Ang N. Optimization of extraction and microencapsulation of
bioactive compounds from red grape (Vitis vinifera L.) pomace (2015) J Food Sci
Technol 52: 783-792. https://doi.org/10.1007/s13197-013-1079-7
48.
Akhavan
Mahdavi S, Jafari SM, Assadpoor E and Dehnad D. Microencapsulation optimization
of natural anthocyanins with maltodextrin, gum arabic and gelatin (2016) Int J
Biol Macromol 85: 379-385. https://doi.org/10.1016/j.ijbiomac.2016.01.011
49.
Saénz
C, Tapia S, Chávez J and Robert P. Microencapsulation by spray drying of
bioactive compounds from cactus pear (Opuntia ficus-indica) (2009) Food Chem
114: 616-622. https://doi.org/10.1016/j.foodchem.2008.09.095
50.
Kalušević
A, Lević S, Čalija B, Pantić M, Belović M, et al. Microencapsulation of
anthocyanin-rich black soybean coat extract by spray drying using maltodextrin,
gum Arabic and skimmed milk powder (2017) J Microencapsul 34: 475-487. https://doi.org/10.1080/02652048.2017.1354939
51.
Ballesteros
LF, Ramirez MJ, Orrego CE, Teixeira JA and Mussatto SI. Encapsulation of
antioxidant phenolic compounds extracted from spent coffee grounds by
freeze-drying and spray-drying using different coating materials (2017) Food
Chem 237: 623-631. https://doi.org/10.1016/j.foodchem.2017.05.142
52.
Cilek
B, Luca A, Hasirci V, Sahin S and Sumnu G. Microencapsulation of phenolic
compounds extracted from sour cherry pomace: effect of formulation,
ultrasonication time and core to coating ratio (2012) Eur Food Res Technol 135:
587-596. https://doi.org/10.1007/s00217-012-1786-8
53.
Akhavan
Mahdavi S, Jafari SM, Assadpoor E and Dehnad D. Microencapsulation optimization
of natural anthocyanins with maltodextrin, gum arabic and gelatin (2016) Int J
Biol Macromol 85: 379-385. https://doi.org/10.1016/j.ijbiomac.2016.01.011
Avocado seeds, extraction of polyphenols,
optimization, response surface methodology.Process Optimization for Extraction of Polyphenols from Avocado Seeds (Persea americana Mill.) Using Response Surface Methodology
Abstract
Full-Text
Introduction
Material and
Methods
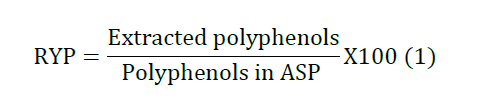
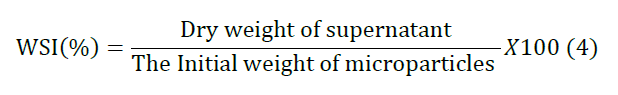
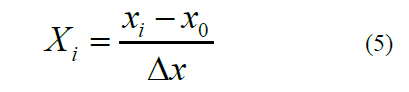
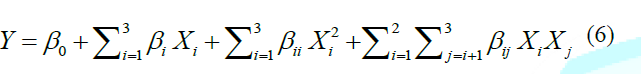
Results
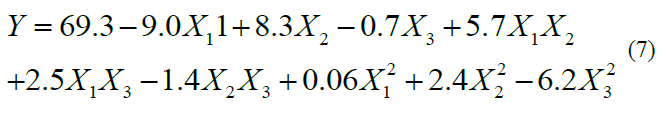

Discussion
Conclusions
References
Corresponding author
Citation
Keywords