Research Article :
Josphert N
Kimatu Cassava (Manihot esculenta), is a major source of carbohydrates after rice
and maize providing a basic diet to over half a billion people. It is an annual
crop belonging to the family Euphorbiaceae. It produces edible root tubers
which form the staple food for inhabitants in the developing world mainly in
the tropical and subtropical countries. It is a very drought tolerant crop
which is classified as either bitter or sweet cassava. However, it produces
Hydrogen Cyanide (HCN) which is toxic. This ant nutritional component can cause
partial paralysis and have been known to kill and wipe out families in Africa.
It is surprising that farmers seem to prefer the bitter varieties as they are
starchier, deter pests and wild animals. There have not been adequate studies
to evaluate the causes and molecular basis of the production of Hydrogen
cyanide by cassava. Observations of feeding patterns of porcupines on cassava
roots, defense mechanisms in cassava and macro level results on cassava
metabolism were hereby used to explain the molecular epigenetic link of
cyanogenesis of cassava. The results explain the exogenous release and its
subsequent removal of HCN during cassava processing. It shall form the basis
for the selection and improvement of cassava products for food security. Cassava (Manihot
esculenta, Crantz), is globally the third largest source of carbohydrates
after rice and maize providing a basic diet to over half a billion people. It
is an annual rustic crop belonging to the dicotyledonous family Euphorbiaceae [1].
It produces edible root tubers which form staple food for inhabitants in developing
world mainly in the tropical and subtropical countries [2]. It is a very
drought and acid soil tolerant crop which although it has thousands of
cultivars, it can be classified as either bitter or sweet cassava [3,4]. The
existence bitterness indicates physical warning of the presence of a poisonous
substance which scientifically has been identified as Hydrogen Cyanide (HCN)
[5]. Although cassava cultivars are clonally propagated through cuttings and
hence are expected to differ little genetically. Studies have surprisingly
however, shown that they have wide variations in HCN concentration ranging from
1 to 2,000 mg/kg [6,7]. The HCN is an ant nutritional component which can cause
partial paralysis and have been known to kill and wipe out families in Africa
[8]. It is surprising that farmers seem to prefer the bitter varieties as they
are starchier, deter pests and wild animals. There have not been many studies
to evaluate the causes and molecular basis of the production of Hydrogen
cyanide by cassava. This study is designed to attempt to do that. The Statement of
the Problem Furthermore, there has not been significant
consensus of the positive correlation between bitterness and HCN level in
cassava. For example, Bokanga and Bradbury, (1994) [9] found an almost
tasteless cassava variety with more HCN (15 mg of HCN per 100g) compared to a
slightly bitter variety with 5mg of HCN per 100g. The problems associated with
the production of cassava HCN are not widespread outside Africa, hence the
causes of high HCN production should be some unique practices done in Africa
[10]. Although, studies have shown that no cassava cultivar, lacks cyanogenic
glycosides because each has a way of protecting itself depending on the level
and frequency of threats [11]. The roots and leaves of cassava contain highest
amounts of two cyanogenic
glucosides referred to as linamarin and lotaustralin [12-14].
The two are broken down by an enzyme called linamarase to produce HCN [15].
However, leaves have higher cyanogenic glycoside levels of 5.0 g linamarin per
kg of fresh weight, whereas roots have about twenty times lower than leaves
[11]. Reconciling the
Experiential and Research Knowledge of Cassava Bitterness Tribes that traditionally consume cassava have come
up with some methods of reducing HCN like soaking, cooking and fermentation,
etc. Such tribes have also great ideas of how the HCN is produced by the
cassava. Previous studies to explore ways to minimize the cyanide content in
cassava and its products had been undertaken but have focused mainly on
agronomic factors. These include genotype or cultivar, stress, soil type,
fertilization, processing techniques, such as cooking, soaking, fermenting and
drying and finally harvest or post-harvest practices such as age at harvest,
housing of products, storage time and temperature. The above should be combined
with other recent advances in plant defense mechanisms and epigenetic studies.
This is can be used to decipher the molecular basis of the cause of HCN
production in cassava and enhance safety is this important diet crop. Hence,
the objective of this study was to explain what actually happens at the
molecular level before the phenotypic observation is made. This shall reconcile
experiential practices with experimental knowledge concerning the molecular
basis of bitterness in cassava. The Foundations
for Unlocking the Mechanisms of HCN Production The cyanogenic glycosides are enzymatically
hydrolyzed by beta-glucosidase as the cassava root tissues are squeezed during
chewing or in the intestine as they are being broken down by gut microorganisms
to release HCN which harmful to the consuming predator or human [16]. Studies have
shown that HCN producing plants should remain relatively free of damage by
general herbivores, but can still be attacked by specialists like porcupines which
have through experience known how to overcome the HCN defenses in cassava
[17-20]. These are herbivores which have devised HCN minimizing mechanisms. In
all studies done, it is becoming clear that cyanogenic glycoside and its
corresponding cyanogenic enzymes are localized in different cellular
compartments or tissues. Therefore, this prevents mixing and cyanogenesis until
the tissues is disrupted [11]. Separation and
Mixing of Glucoside and Linamarin in Cassava In some plants, the separation of the substrate and
cyanogenic enzymes is at the subcellular level while in others like sorghum is
at the tissue level [21]. For example, in rubber trees, the endosperm contains linamarin but
the linamarase is located in the apoplast [22,23]. In cassava leaves, linamarin is located in the
vacuoles, while the enzyme linamarase is localized to cell walls and laticifers
almost 8-fold [24-27]. These results suggest that for HCN to be released there
should be a mechanical disruption strong enough to trigger the mixing. Previous
studies have shown that linamarin and its β-glucosidase, linamarase, are
actually present in all cassava organs except seeds. An explanation of why this
substance is located in the cell wall is that it serves as signal to detect and
transmit any significant physical interference from exogenous attackers who are
trying to gain entry into the cell. However, if they enter the cell or a strong
enough, then the cell triggers another chemical defense against them
endogenously. This seems to be similar to the two lines of defense of animals cells against pathogens, the latter being
antibodies production. The cassava peel which account for 11-20% of the root
weight is made up of sclerenchyma and phloem cells; it has a high amount of
cyanogenic glycoside and is therefore removed during cassava processing by
almost all consumers [28]. The nature and amount of preformed pathogen inhibitors
are influenced by the environment, genotype and age of the plant [29,30]. The Possible
Epigenetic Link of HCN Release in Cassava Studies by White, et al. [11] suggested that, the
molecular basis for the absence of hydroxynitrile lyase, which catalyzes the
last step to release HCN from roots and stems could be attributed to very low
steady-state hydroxynitrile
lyase transcript levels (relative to leaves), suggesting
that hydroxynitrile lyase expression is regulated at a pretranslational level.
However, later studies confirmed that the mechanical disruption could be
responsible for its release, for example, it could be found in leaves which are
always disturbed compared to stem and leaves in studies in cassava, sorghum and
flax (Linum usitatissimum) [31].
Later studies could however not fully establish whether linamarin is
transported apoplastically between shoots and roots or between root cells [11]. Other similar studies further point to the
epigenetic expression due to biotic stress, for example, the expression of the
rice per gene is induced during fungal infection. Plants seems to release the
enzymes based on some epigenetic memory of the stress using epigenetic
processes, like include inherited DNA methylation and histone modifications, in
subsequent cassava generations [32,33]. Epigenomic control modulates gene
expression in response to environmental stimuli through signal transduction and
other rapid defenses responses. Cassava has been classified as sweet and bitter
cultivars; this demarcation can also be related to the production of defense
chemicals (cyanogenesis) by
the plants against herbivores and pathogens at the same time. In places with
mixed farming the cassava plant might be in close proximity with other plants
which attract many microbes, this might make the cassava to produce more
defense chemicals than one which is grown in monoculture systems. A cassava in
poor soils or harsh environment might also be targeted by pathogens and hence it
might produce more toxic defense chemicals. This might explain some cases of
cassava poisoning in East Africa region compared to West African region. The Rapid
Response of Cassava to Abiotic Stresses The cassava plant opens its stomata only at low
evaporation demand and when water use efficiency is highest. The leaves show
heloitropic responses making it to obtain maximum light. The leaves also droop
at bright noon light to protect it from excess UV light [34]. It is no surprise
if it has internal mechanisms to protect itself from predators including root
pathogens like Phytophthora Root
Rots
(PRR) Phytophthora spp. [35]. Cyanogenic glycosides are used by many plants to
defend themselves [36]. They also regulate the plant-insect interactions [37].
There are at least 2500 species of plants that produce cyanogenic glycosides
and a corresponding hydrolytic enzyme called beta-glycosidase. The plant-predator
protection mechanism occurs when the two produce a sugar and a cyanohydrin
which rapidly decomposed to HCN and an aldehyde or a ketone. Three, glycosides,
cyanohydrins and hydrogen cyanide are known as cyanogens. Recent studies have also associated production of
HCN to abiotic stresses like dry spells which encourage water stress, increased
weeds in the cassava farm, soil characteristics (which might mean deficiency or
toxicity of particular elements), the age of the plant, piece meal mechanical
harvesting (indicating disturbance of ground around cassava plants) and cassava branch
pruning [38]. The Method of
Harvesting and Hydrogen Cyanide Production Cassava is generally manually harvested. The stems
are cut off 40-60 cm above the soil so that the stem portion can be handled
when uprooting the tubers. In other cases, harvesting involves digging up the
roots [3]. The correlation between the harvesting method and amount of HCN has
not so far been investigated. Area of Study This study was done at Mua Hills in Machakos County,
in Kenya, East Africa, which lies at latitude: 1.45 South and longitude: 37.21
East with an altitude up to 1967.00m/6453.41ft. The area has red soils which
are loosely packed. The cassava is grown in ridges of soils. The temperatures
are between 18-25ºC in a day. The area is near a game reserve with
numerous wild animals including nocturnal animals like the African
porcupines (Hystrix
cristata). The lower side towards Kapiti plains usually has
farmers planting cassava but the farmers are harassed by porcupines from the
nearby game reserve. The study aimed at providing molecular explanation of the
macro level experimental results of cassava studies with experiential practices
by farmers and consumers by utilizing plant molecular epigenetic findings.
Systematic observation on nocturnal predators on cassava was done. These
predatory patterns of porcupines on cassava were recorded observation in form of
photos for detailed analysis. The study also focused on literature on cassava
on world wide scale and analyzed and explained some previous results based on
recent findings and observations. Porcupine
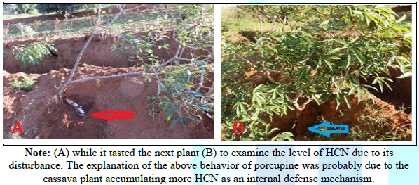
Studies on Cassava HCN Our studies with porcupines and cassava farms at the
border between the in Mua Hills and the Kapiti plains showed that when a
porcupine dug the roots of a particular cassava and consumed a portion of its
root, the porcupine did not return to the same plant the next day. These puzzled researchers, who thought that the
economics of rejecting ready food and sacrificing to dig for fresh one could,
have meant a life and death affair. The most likely explanation was that the
cassava produced a fatal HCN to deter the porcupine from coming to back to the
same plant to finish its left-over food. Furthermore, the porcupine could have
the ability detect the presence of HCN. This could be via the sniffing and
detecting of high dangerous concentrations of HCN. This detection made it to go
and dig another plant further from the first one. This is because ground
disturbance could trigger HCN production in neighboring cassava plants (Figure 1). Portion of Cassava root left by a porcupine. Figure 1: Portion of Cassava root left by aporcupine. Studies on
Cassava Farms Our studies showed disturbed cassava farms produced
bitter roots, for example when animals passed through a cassava farm the
cassava dug from the farm where mostly bitter, when one dug cassava roots
immediately after a shower the roots where majorly bitter, also when one hung
clothes on cassava plants and latter dug the roots the plants had bitter roots.
Surprisingly, when small children struggled to get a cassava root and spent
more time trying to get the root out, the roots turned to be bitter. Our literature studies found out that two families
of Makueni District and another of Kathonzweni District, in Kenya were affected
after consuming raw and cooked cassava in August and September 2011. A
4-year-old child died in the first family, the family looked extremely poor and
the only meal they had was boiled and raw cassava. In the second family, a
child aged 5 died in Makueni District Hospital while continuing with HCN
poisoning management. Both families complained of headaches, abdominal pains
and discomfort vomiting, general body weakness and some fever. The Health
Officer collected the cooked and uncooked cassava and fresh samples from the
same plants where cassava was harvested. The cassava tasted bitter as claimed
by the family members. The area had experienced drought for the last 3 years
[39]. Time Required
for a Cassava to Produce HCN In removing HCN people usually soak the root tubers
for 4 hours but that is not sufficient, only 18-24 hours can reduce HCN by 50%.
A dose of 0.5 to 3.5 mg per kg of human body weight is enough to show HCN
intoxication symptoms like rapid respiration, low blood pressure, headache and
dizziness, intestinal pain, vomiting and diarrhea and can result in death. The
mechanical shaking of a cassava plant is easily transferred to the roots as the
plant has heavy leaves which are close to the ground where the tubers are
formed (Figure 2). HCN Studies on
Food Products Samples from Vanuatu had HCN levels of 26 mg/kg to
78 mg/kg but the flour sample from the same had more cyanide content of 57
mg/kg while the cassava chips had 60 mg/kg. Cassava is stored under ambient
temperatures, the cyanide levels drops
by about 30% after four days [39]. Other studies in Africa showed a seasonal
variation in cassava HCN levels with higher levels in dry conditions with the
cassava becoming bitter. The cyanide content was found to be higher in younger
leaves compared to older ones [40]. Time Dependent Production
of HCN during the Harvest Period of Cassava The production of Cyanogenesis in cassava can be
seen first as a static protection offered by a particular cultivar s
constitutive level of cyanogenic glycosides which causes it to have a certain
level of bitter taste. Secondly, it can be viewed as a rapid formation of HCN
during a mechanical disturbance or a feeding episode by chewing animals or
insects on leaves. The first production of bitter glucosides is
cultivar, level of growth and other environmental factors, but the second one
is a kind of an epigenetic regulation which is rapid as catalyzed by endogenous
enzymes to produce HCN. This can help us to understand why
some cassava cultivars are mildly bitter but may not be toxic at the level of
HCN production depending of the level of disturbance just before harvesting. Some
specialized predators like insects have enzymes that transform cyanogenic
glycosides into harmless substance in their gut or may hinder the conversion of
glucosides in to HCN in their gut, while those who are not adapted to the toxic
have to chew it in a way that make it to release the HCN which is lost into the
atmosphere before they swallow [41]. Human being depends of the second method
to mechanically reduce the HCN before consuming. Therefore, the various processing techniques of cassava
significantly reduces the toxicity of HCN because studies have shown that the
proportion of HCN, diffused and ingested, will depend on HCN evolution by the
plant s tissue, the speed at which the root tuber is eaten. HCN is also harmful
to the plant; therefore, it must be produced at a rapid speed at the time of
attack or disturbance. Other noncyanogenic amino acid precursors have been used
by plants to deter predators during seed germination and early seedling growth.
This phenomenon has been observed in other plants
for example in Pteridium arachnoideum, Eucalyptus polyanthemos and in the
legume Phaseolus lunatus [42-44]. The number of cyanogenic glycosides varies in
different plant tissues, organs, species and environmental conditions where it
grows [45]. Normally, human beings have acidic stomach environments that
deactivate the β-glucosidase enzyme making the production of HCN not possible.
How it is that diversification of diet may reduce HCN poisoning? In humans, HCN
is detoxified by the enzyme rhodanese, forming thiocyanate, which is excreted
in the urine. However, this detoxification used Sulphur donors,
which are derived from Sulphur amino acids from the protein rich food consumed
[46-48]. The Epigenetic
Link of HCN Production and Food Security The above studies strongly suggest that cassava uses
an internal molecular mechanism to protect itself from herbivores and other
enemies. It stays alert by preparing a precursor for HCN which through a rapid
epigenetic mechanism it triggers an expression of the genes of HCN metabolic
pathway when mechanically disturbed. Some cassava plants do not find it
necessary to constantly produce the HCN precursor because they are in favorable
conditions for a long time. Hence, as we plan to utilize this dry land resource
for food security, we should be aware of this by avoiding the HCN from the
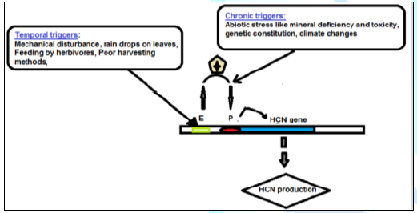
cassava plant through careful harvesting, processing and habitat selection (Figure 3). Figure 3: The postulated molecular epigeneticcontrol of HCN production at the gene level. Temporal HCN production caused mainly by
external mechanical factors act on upstream Enhancers (E) of the DNA. This
could be via simple nucleosome configurations. When this stimulation passes a
certain threshold, in respect of minimizing metabolism expenditure, it triggers
transcription factors to regulate cytosine methylation of demethylation causing
the activation of Promoters (P)
which now increases the internal chronic HCN production to cause bitterness in
a cassava variety.
Kimatu JN. Possible causes and the molecular basis
of hydrogen cyanogenesis production in cassava (2020) Edelweiss Food Sci
Tech 1: 27-31. Food security, Processing, Value
addition, Starch, Ethanol, Cuttings.Possible Causes and the Molecular Basis of Hydrogen Cyanogenesis Production in Cassava
Abstract
Full-Text
Introduction
Method
Results
Discussions
and Conclusions
References
*Corresponding author
Josphert
N Kimatu, Department of Life Sciences, South Eastern Kenya University, Research
and Innovation Center, Kitui, Kenya, Tel: +2547050521571, Email: jkimatu@seku.ac.ke
Citation
Keywords