Research Article :
Cefuroxime
axetil (CA) a pro-drug was tested as corrosion inhibitor for aluminum in
hydrochloric acid solution using thermometric, gasometric weight loss and
scanning electron microscope (SEM) techniques. Results obtained showed that
this compound has good inhibiting properties for aluminum corrosion in acidic
medium, with inhibition efficiencies values reaching 89.87 % at 0.5 g / L. It
was also found out that the results from weight loss method are highly consistent
with those obtained by hydrogen evolution method and gasometric method; and all
indicate that inhibitor efficiency increases with increasing inhibitor
concentration. Cefuroxime axetil inhibited the corrosion of aluminum in
solutions of HCl through the mechanism of physiosorption as confirmed by values
of activation energy and free energy of adsorption. The adsorption of the
inhibitor was also found to be spontaneous, exothermic and best fitted the
Langmuir adsorption model. SEM analysis confirmed the existence of an absorbed
protective film on the aluminum surface. In developed and developing
countries, billions of dollars every year are spent on capital replacement and
control methods for corrosion infrastructure [1,2]. In recent years, owing to
the growing interest and attention of the world towards the protection of the
environment and the hazardous effects of using chemicals on the ecological
balance, the use of eco-friendly inhibitors to replace the older, which is more
toxic and harmful to the environment are been intensified [3-5].
Research has shown that for an
inhibitor to be an effective protector against metal corrosion, it should be
readily adsorbed on the metal surface through either physisorption or
chemisorption processes [6-11]. Either of these adsorption processes depends
primarily on the physicochemical properties of the inhibitor group such as
functional groups, electronic density at the donor atom, molecular structure,
etc. For instance, organic molecules, which have had a wide applicability and
that have been extensively studied and used as corrosion inhibitors, often
contain the hetroatoms nitrogen, oxygen, and sulfur atoms, as well as multiple
bonds in their molecules. Several researches have been carried out on the use
of drugs as corrosion inhibitors for several metals in various media. For
example, Fouda et al., studied the
corrosion inhibition characteristics of floxacillin, cloxacillin,
dicloxacillin, cefadroxil and cephalexin on aluminum in 0.5 M H3PO4 using
weight loss and galvanostatic polarization techniques
Results
obtained revealed that the inhibition occurs through adsorption of the
inhibitor molecules on the metal surface.
Other
drugs that have been found to be good corrosion inhibitors include norfloxacin,
Streptomycin, Cefatrexyl, and Cefazolin [13-15]. The choice of some of the
drugs used as corrosion inhibitors may be due to the fact that they have a
large number of functional adsorption centers, are biodegradable, can be easily
produced and purified.
In the present investigation, the corrosion inhibiting behavior of
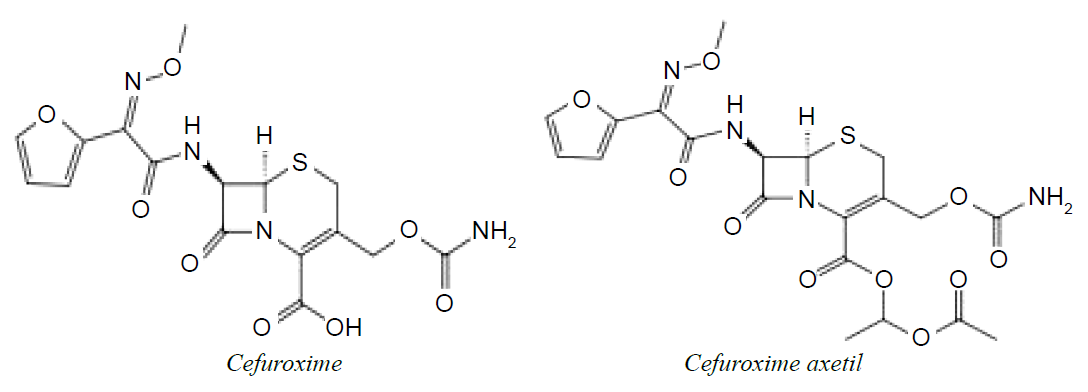
Cefuroxime axetil [Chemical name: 1-Acetoxyethyl (6R,7R)-3-{(aminocarbonyl)oxy]methyl}-7-{[(2Z)-2-(2-furyl)-2-(methoxyimino)
acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate] was investigated on aluminum in hydrochloric acid at 303
and 333 K using weight loss, thermometric gasometric and scanning electron
microscope techniques. Cefuroxime is a broad-spectrum,
β-lactamase-stable second generation cephalosporin antibiotic drug with
well-defined pharmacokinetics after injection [16]. It is commercially available
for parenteral (intramuscular and intravenous) administration as a sodium salt
and for oral administration as cefuroxime axetil, the 1-(acetoxy)ethyl ester of
the drug [17]. In humans, gastrointestinal absorption of cefuroxime is
negligible, whereas cefuroxime axetil shows a bioavailability of 30 to 40% when
taken on fasting and 50 to 60% when taken after food [18-23]. In fact like the
other cephalosporins, the parenteral cefuroxime, is not orally absorbed due to
the presence of a highly polar carboxyl group that is ionized at intestinal pH,
making transport across intestinal mucosa unlikely [24,25]. Cefuroxime axetil
is a prodrug of cefuroxime and has little, if any, antibacterial activity until
hydrolyzed in vivo to cefuroxime. The cefuroxime axetil esterase can hydrolyze
cefuroxime axetil to the non-absorbable cefuroxime in the gut lumen [26].
Esterase hydrolysis leads to ethanoic acid and an unstable hydroxyethyl ester,
which dissociates rapidly to ethanol and the parent cefuroxime [27]. Figure 1
shows the chemical structures of both cefuroxime and cefuroxime axetil.
The
selection of cefuroxime axetil as a corrosion inhibitor is based on the
following facts:
1.
It
contains three kinds of heteroatoms (four nitrogen, ten oxygen and one sulphur
atom) as reactive center through which they can adsorb readily on the metal
surface. 2.
The
compound is readily soluble in medium. 3.
It does
not cause any health hazards, but also find its diverse applications in various
biological and pharmacological activities; hence the use of Cefuroxime axetil
as corrosion inhibitors is safe. A previously weighed metal
(aluminum sheet) was completely immersed in 250 ml of the test solution in an
open beaker. The beaker was inserted into a water bath maintained at a
temperature of 30 °C. Similar experiments were repeated at 60°C. In each case,
the weight of the sample before immersion was measured using Scaltec high
precision balance (Model SPB31) After every 24 Figure 1: Chemical structures of Cefuroxime and Cefuroxime
axetil. hours, each sample was removed from the test solution, washed in a
solution of NaOH containing zinc dust and dried in acetone before re-weighing.
The difference in weight for a period of 168 hours was taken as total weight
loss. The inhibition efficiency (% I) for each inhibitor was calculated using
equation (1) [28] Where, W1 and W2 are the
weight losses (g/dm3) for mild steel in the presence and absence of
inhibitor in HCl solution respectively. The degree of surface coverage θ is
given by the equation (2) [29]:
The corrosion rates for mild
steel corrosion in different concentrations of the acid was determined for 168 h
immersion period from weight loss using equation (3) [6] Where, W = weight loss (mg); D =
density of specimen (g/ cm3), A = area of specimen (square inches) and T = period of immersion
(hour).
Gasometry method The method used for hydrogen
evolution measurement is as described elsewhere [30]. The test solution
(different concentrations of acid, inhibitor or their mixtures) was poured into
the reaction vessel (gasometer). Upon the introduction of mild steel, the flask
was quickly corked and the rise in volume of the paraffin due to hydrogen
evolution was noted after every minute until a steady volume was observed. From
the results obtained, the corrosion inhibition efficiency was calculated using
the following equation,
Where, Vb is the
volume of hydrogen gas evolved by the blank and Vt is the
volume of hydrogen gas evolved in the presence of the inhibitor, after time, t.
This was also carried out as reported elsewhere
[31]. The reaction number (RN) of each system was calculated by dividing the
difference between the highest and lowest temperature attained by the time
interval. From the reaction number, the inhibition efficiency (% I) of the
inhibitor was calculated using equation (5)Where, RNaq is the
reaction number in the absence of inhibitors (blank solution) and RNwi is the reaction number of 2 M HCl containing the
studied inhibitor.
Scanning electron microscopy The scanning electron microscope (SEM) Model No
JSM-5600 LV was used to study the morphology of corroded in the presence and
absence of inhibitor. The photographs were taken from that portion of the specimen where better information was expected.
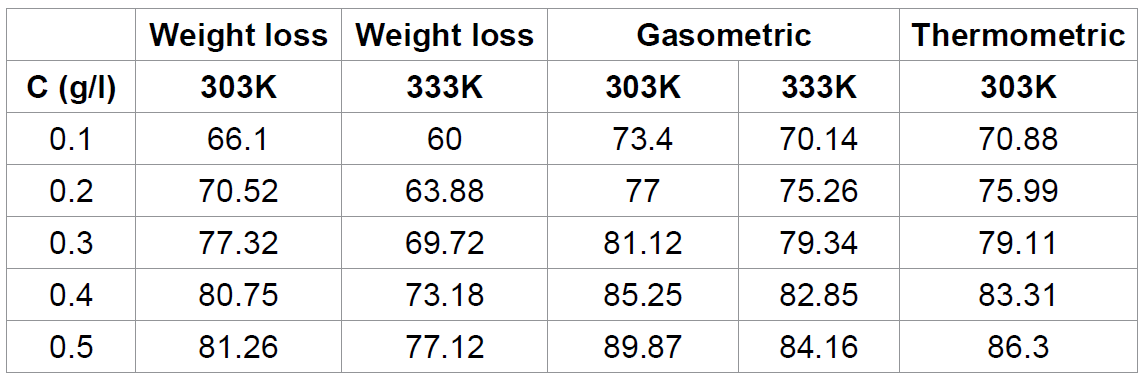
Table 2. presents inhibition efficiencies of
various concentrations of Cefuroxime axetil in HCL. The inhibition efficiency
was estimated to be 73.40 % even at extremely low inhibition concentration (0.1
M) and reaches 89.87 % at a concentration of 0.5 M. Such remarkable
performances may be due to, the high molecular weight of CA, the presence of
C=N, O-H, C=O, etc. which are electron donation groups and the presence of aryl
groups. The inhibition efficiency was found to increases with increase in the
concentration of the inhibitor but decreases with increase in temperature
indicating that the mechanism of physical adsorption favours the adsorption of
Cefuroxime axetil on aluminum surface. For a physical adsorption mechanism, the
inhibition efficiency is expected to decrease with increase in temperature but
for a chemical adsorption mechanism, the reverse is expected [31].
Also, the inhibition efficiency of CA obtained from the two methods were
found correlate strongly (R2 = 0.8798
and 0.9644 for gasometric and thermometric respectively) with those obtained from gravimetric method. However, values of
inhibition efficiency obtained from the weight loss were higher than the values
obtained from thermometric and gasometric methods indicating that the average
inhibition efficiency of CA is better than its instantaneous inhibition
efficiency.
Table
2: Inhibition efficiencies of Cefuroxime axetil for the corrosion of aluminum
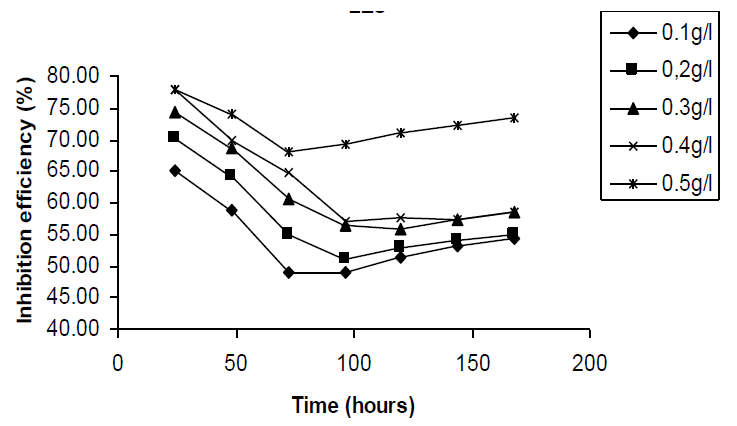
in HCL The stability of the inhibitive properties of the
studied inhibitor over 168 hours of immersion was studied by plotting values of
inhibition efficiencies gotten from weight loss studies against time as shown
in Figure 2. From the plots, it is also evident that the inhibition efficiency
first decreased with time until a critical value was attained after which it
started increasing. This trend suggests that at first, there was competition
between the forces of adsorption and desorption and that after the critical
zone, adsorption facilitated the formation of a protective layer and thus
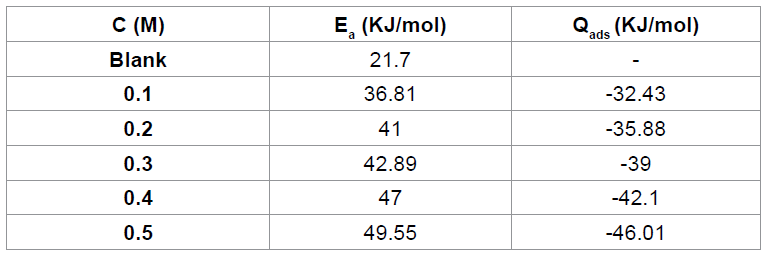
protected the metal against further corrosion attack. Effect of temperature According to Eddy [32],
temperature affects the rate of any chemical reaction such that an increase in
temperature leads to a corresponding increase in the rate of the reaction.
Hence the effect of temperature on the corrosion of aluminum in HCl (in the
absence and presence of CA) was studied using the Arrhenius Equation (equation
6) where the apparent activation energies (Ea) for the corrosion process in absence and presence
of inhibitor were evaluated [6,33].
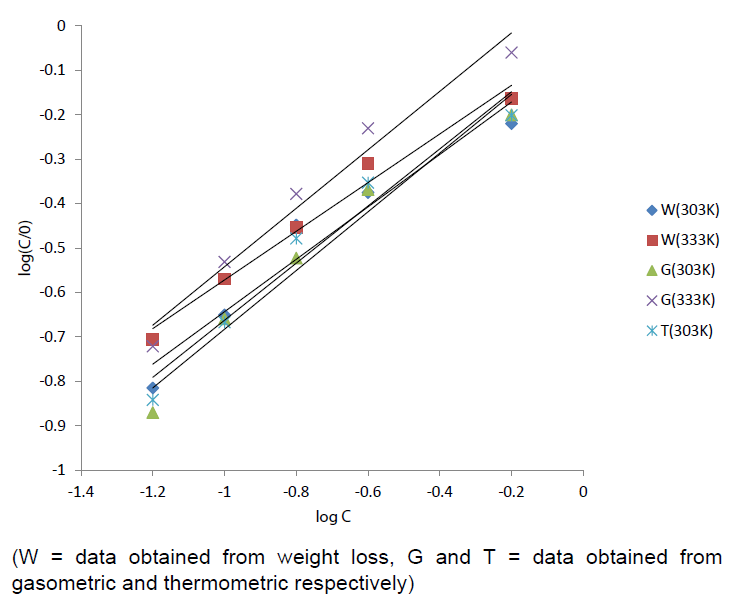
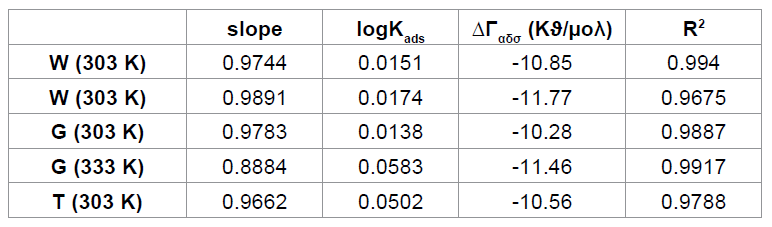
Figure 3: Langmuir isotherm for the adsorption of Cefuroxime
axetil on the surface of aluminum. Table
3: Activation energy and heat of adsorption of Cefuroxime axetil on the surface
of aluminum Table
4: Langmuir parameters for the adsorption of Cefuroxime axetil on the surface
of aluminum. Where, CR1 and CR2 are the corrosion rates of aluminum in solution of HCl at the
temperatures, T1(303 K) and T2 (333 K) respectively, Ea is the activation energy for the adsorption of CA on Al surface and R
is the gas constant. Calculated values of the Ea are shown in Table 3. It can be
seen from the table that the activation energies are lower than the value of 80
kJmol-1 required for a chemical
adsorption mechanism supporting the earlier claims the adsorption of CA on
aluminum proceeds by physical adsorption mechanism. Conclusions From the study, the following
conclusions can be drawn. CA efficiently inhibits the corrosion of mild steel
in 0.1M HCl medium. Adsorption of CA on the surface of aluminum from 1M HCl
obeys Langumur adsorption isotherm. The inhibition efficiency of CA increases
with increasing the inhibitor concentration and on increasing the temperature,
the corrosion rate increases. The calculated values of Ea, Qads, ∆Gads indicates that the adsorption of
inhibitor on the metal surface
The
authors are grateful to TEFUND for sponsoring the research and Mrs Janet Onoja
Ameh for typesetting the manuscript.
1. Thompson
NG, Yunovich M, Dunmire D. Cost of corrosion and corrosion maintenance
strategies (2007) Corros Rev 25:247–262. 2. Cherry
BW, Skerry BS, Clayton Vic. Corrosion in Australia: the report of the
Australian National Centre for Corrosion Prevention and Control feasibility
Study (1983) Department of Materials Engineering, Monash University:Australia. 3. Patni
N, Agarwal S, Shah Pallav. Greener approach towards corrosion inhibition (2013)
Chin J Eng 2013:1–10. 4. Dalo
Abu AM, Othman AA, Rawashdeh Al FAN. Exudate gum from Acacia trees as green
corrosion inhibitor for mild steel in acidic media (2012) Int J Electrochem Sci
7:9303–9324. 5. Sharma
SK, Mudhoo A, Khamis E. Adsorption studies, modeling and use of green
inhibitors in corrosion inhibition: an overview of recent research, green
corrosion inhibitors: status in developing countries (2011) Green corrosion
chemistry and engineering. Wiley–VCH Publications:319. 6. Ameh
PO, Oyeniyi S, Qand Sani UM. Quantum Chemical Studies on the Corrosion
Inhibitions of Mild Steel in Acidic Medium by
5-amino-1-cyclopropyl-7-[(3R,5S)-3,5-dimethylpiperazin-1-yl]-6,8-difluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic
acid (2015) International Journal of Chemical, Material and Environmental
Research 2:1-16. 7. Abdel
Hameed RS. Expired drugs as corrosion inhibitors for metals and alloys (2013)
Journal of physical chemistry PCAIJ 8:146-149. 8. Deepa
Rani P, Selvaraj S. Inhibitive action of Vitis vinifera (grape) on copper and
brass in natural sea water environment (2010) Rasayan J Chem 3:473-482. 9. Saratha
R, Kasthuri N, Thilagavathy P. Environment friendly acid corrosion inhibition
of mild steel by Ricinus communis Leaves (2009) Der Pharma Chemica1 2:249-257. 10. Satapathy AK, Gunasekaran G, Sahoo SC, Kumar Amit Rodrigues
PV. Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric
acid solution (2009) Corros Sci 51:2848–2856. 11. Morad MS. Inhibition of iron corrosion in acid solutions by
Cefatrexyl, Behaviour near and at the corrosion potential (2008) Corros Sci
50:436-441. 12. Fouda AS, Al-Sarawy AA, Ahmed FS, El-Abbasy HM. Corrosion
inhibition of aluminum 6063 using some pharmaceutical compounds (2009)
Corrosion Science 51:485–492. 13. Eddy NO, Odoemelam SA. Adsorption and inhibitive properties
of norfloxacin for the corrosion of mild steel in H2SO4 (2008) Internat J of
Pure and Applied Chem 3:1-10. 14. Shukla SK, Singh AK, Ahamad I, Quraishi MA. Streptomycin: A
commercially available drug as corrosion inhibitor for mild steel in
hydrochloric acid solution (2009) Materials Letters 63:819. 16. [McEvoy GK. Cephalosporins: cefuroxime sodium and
cefuroxime axetil (2003) AHFS Drug Information, American Society of Hospital
Pharmacists, Wisconsin Avenue:223-231. 17. Nieves Ruiz-Balaguer, Amparo Nacher, Vicente G. Casabo, and
Matilde Merino (1997). Nonlinear Intestinal Absorption Kinetics of Cefuroxime
Axetil in Rats. ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Vol. 41, No. 2: 445–448. 18. Ridgway E, Stewart K, Rai G, Kelsey MC, Bielawska C. The
pharmacokinetics of cefuroxime axetil in the sick elderly patient (1991) J
Antimicrob Chemother 27:663-668. 19. Wozniak T, J Hicks. Cefuroxime sodium (1991) Anal Profiles
Drug Subst 20:209–237. 20. Sommers DK, van Wyk M, Moncrieff J, Schoeman HS. Influence
of food and reduced gastric acidity on the bioavailability of bacampicillin and
cefuroxime axetil (1984) Br J Clin Pharmacol 18:535-539. 21. Finn A, Straughn A, Meyer M, Chubb J. Effect of dose and
food on the bioavailability of cefuroxime axetil (1987) Biopharm Drug Dispos
8:519-526. 23. Williams PE, Harding SM. The absolute bioavailability of oral
cefuroxime axetil in male and female volunteers after fasting and after food
(1984) J Antimicrob Chemother 13:191-196. 24. Foord RD. Cefuroxime:human pharmacokinetics (1976)
Antimicrob Agents Chemother 9:741-747. 25. Tsuji A, Hirooka H, Tamai I, Terasaki T. Evidence for a
Carrier-Mediated Transport System in the Small Intestine Available for FK089, a
New Cephalosporin Antibiotic Without an Amino Group(1986) J Antibiot 39:1592–
1597. 26. Harding SM. Comparative pharmacokinetics of tablets and
suspension (1990) Res ClinForums12:23-29. 27. Bundgaard H. Design of Prodrugs: Bioreversible Derivatives
for Various Functional Groups and Chemical Entities (1985) Design of Prodrugs,
NY Elsevier Science Publishing Co:1–92 28. Oguzie EE. Evaluation of some inhibitive effect of some
plant extracts on the acid corrosion of mild steel (2008) Corros Sci
50:2993-2998. 31. Umoren SA, Ogbobe O, Ebenso EE, Okafor PC. Polyethylene
glycol and polyvinyl alcohol as corrosion inhibitors for aluminium in acidic
medium (2007) J Appl Polym Sci 105:3363-3370. 33. Momoh Yahaya H, Eddy NO, Iyun JF, Gimba CE, Oguzie EE.
Inhibitive and Adsorptive Behaviour of Guanine on Corrosion of Mild Steel in
0.1M HCl and H2SO4 (2012) International Journal of Modern Chemistry 2:127-142. 34. Bhajiwala HM, Vashi RT. Ethanolamine, Diethanolamine and
Triethanolamine as Corrosion Inhibitors for Zinc in Binary Acid Mixture
[HNO3+H3PO4] (2001) Bull Electrochem Soc 17:441-448. 35. Eddy NO, Ibok UJ, Ebenso EE. Adsorption, synergistic
inhibitive effect and quantum chemical studies on ampicillin and halides for
the corrosion of mild steel (2010) Journal of Applied Electrochemistry
40:445-456. 36. Ghasemi Z, Tizpar A. The inhibition effect of some amino
acids towards Pb Sb–Se–As alloy corrosion in sulfuric acid solution (2006) Appl
Sci 252:3667– 3672. 37. Flis J, T Zakroczymski. Impedance study of reinforcing
steel in simulated pore solution with tannin (1996) Journal of the
Electrochemical Society 143:2458–2464. 38. Bilgic S, Sahin M.The corrosion inhibition of austenitic
chromium–nickel steel in H2SO4 by 2-butyn-1-ol (2001) Mater Chem Phys
70:290–295. 39. Rafiquee MZA, Khan S, Saxena N, Quraishi MA. Influence of
Some Thiadiazole Derivatives on Corrosion Inhibition of Mild Steel in Formic
and Acetic Acid Media (2007) Portugaliae Electrochimica Acta 25:419–430.Cefuroxime Axetil: A Commercially Available Pro-Drug as Corrosion Inhibitor for Aluminum in Hydrochloric Acid Solution
Abstract
Full-Text
Introduction
Materials and Methods
Weight loss method
Thermometric method
Results and Discussions
Effect of cefuroxime axetil
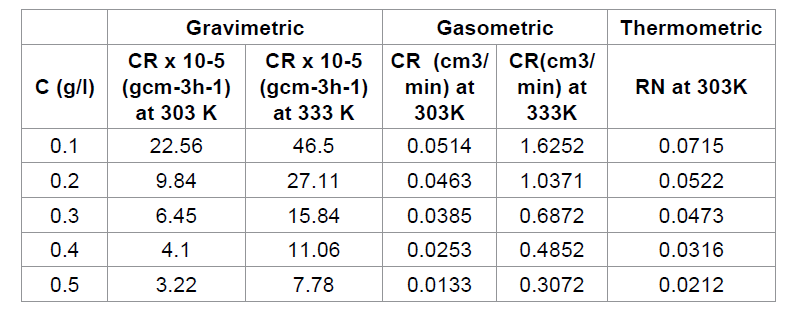
The corrosion rate (CR) and reaction number (RN) of
aluminum in HCl containing various concentrations of Cefuroxime axetil
determined for 168h immersion period from gravimetric, gasometric and
thermometric are presented in Table 1. The results obtained indicates that the
aluminum corrosion is reduced by the presence of Cefuroxime axetil in HCL at
all concentrations used in this study, since there is a general decrease in the
rate of corrosion of the aluminum with increase in concentration of the
inhibitor. This may be ascribed to the adsorption of this compound on the
aluminum, producing a barrier, which isolates the surface from the corrosion
environment.
Acknowledgment
References