Commentary :
The rationale behind targeted drug
delivery is to produce a system that can deliver drugs at rates finely tuned to
the biological requirements of the body [6] with high specificity and efficacy [1].
The primary objective is to develop a system that protects the payload and
improves the therapeutic index [8,9]. In this regard, gold
nanoparticles (AuNPs) have come into the spotlight of targeted
pharmaceuticals. Amongst the wide range of nanomaterials
used for anticancer therapy, AuNPs hold tremendous importance
[7] due to their unique ability to respond to a variety of different stimuli,
such as molecular binding or changes in ionic concentration, and release cargo
instantaneously [10]. AuNPs can also be combined with targeting ligands in
roder to reach sub-cellular compartments in specific tissue(s) [8]. Targeted drug
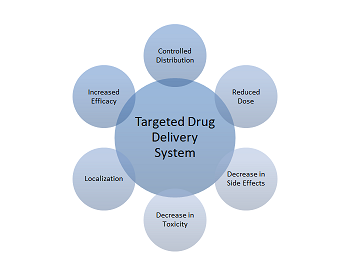
delivery aims at increasing therapeutic efficacy, achieving controlled
distribution, improving drug localization and reducing drug toxicity (Figure 1)
[1]. In this regard, metallic nanoparticles
offer a new dimension towards the fulfillment of these aims in treating various
diseases and their simplicity and ease of preparation has precipitated their
interest in the scientific community [2]. Diseases such as cancer as well as several
ocular diseases display many similarities in potential nano-based therapeutic
intervention owing to their unusual chemistry and a variety of design
considerations. Their physiochemical similarities, such as the
over-expression of angiogenic factors, have inspired the
design and development of pharmaceutical agents for targeted delivery where the
drugs can safely reach their targets and deliver the cargo at the site of need
with little to no interaction to surrounding structures and cells [3]. In
addition to this, metallic nanoparticles with magnetic properties can also be
used as drug delivery agents while under the influence of a magnetic field [4].
When looking specifically at ovarian cancer, the delivery of siRNA in a nanoscale
metallic framework along with cisplatin manifests tremendous potential in re-sensitizing
ovarian tumor cells to chemotherapy [5]. The application of these frameworks in
conjunction with photodynamic therapy promises a great deal from the standpoint
of cancer targeting as well ocular disease therapeutics [6,7]. The rationale behind targeted drug
delivery is to produce a system that can deliver drugs at rates finely tuned to
the biological requirements of the body [6] with high specificity and efficacy [1].
The primary objective is to develop a system that protects the payload and
improves the therapeutic index [8,9]. In this regard, gold
nanoparticles (AuNPs) have come into the spotlight of targeted
pharmaceuticals. Amongst the wide range of nanomaterials
used for anticancer therapy, AuNPs hold tremendous importance
[7] due to their unique ability to respond to a variety of different stimuli,
such as molecular binding or changes in ionic concentration, and release cargo
instantaneously [10]. AuNPs can also be combined with targeting ligands in
roder to reach sub-cellular compartments in specific tissue(s) [8]. A class of peptides, called integrins,
is known to mediate intracellular signaling and gene expression. In recent
studies, their role as highly amenable target molecules for cancer therapy has
become evident [9]. Conjugation of these integrin peptides to the surfaces of
AuNPs, as well as other metallic nanoparticles, holds significant promise in
anticancer medicine [11]. Moreover, integrin targeted radiotracers can be used
in tumor imaging by single photon emission computed tomography (SPECT) [12]. As
integrins are prime targets for synaptic drug delivery, they can be
functionalized on the surface of AuNPs to also provide theranostic application [1].
On the other hand, research in ocular
disease treatment has also witnessed a significant perusal of AuNPs.
Their self-therapeutic properties were utilized by Song et al to inhibit vascular angiogenesis in mice with oxygen-induced
retinopathy. The surface properties of these nanoparticles were fine-tuned for
Optical Coherence Tomography (OCT) imaging, and showed significant suppression
of Vascular Endothelial Growth Factor (VEGF) in vivo [13]. Also, AuNPs designed by Karthikeyan et al demonstrated promising blockage of
VEGF-induced cell proliferation in bovine retinal pigment epithelial (RPE)
cells [14]. Additionally, VEGFR2 suppression in animal models of retinopathy of
prematurity (ROP) via AuNPs, designed by Kim et al, provides promising evidence of the effectiveness of
gold-based nanoparticles in the area of ocular disease treatment [15]. Table
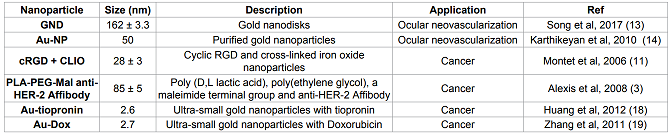
1:
Examples and characteristics of nano-based drug delivery systems to treat various
diseases (i.e. cancer and ocular neovascularization). Figure 1: Ideal characteristics of a nano-based targeted
drug delivery system. Magnetic
Nanoparticles (MNPs) From the standpoint of biomedical
imaging, the behavior of magnetic nanoparticles (MNPs) is affected
by size, shape, surface defects and coating [2]. They provide a non-invasive
means of achieving biological control at the nanoscale. The category of MNPs includes
metallic, bimetallic and superparamagnetic iron oxide nanoparticles (SPIOs). Alongside
their tunable magnetic properties, MNPs can be made to target tissues via
biocompatible coatings [16]. One can also purposely increase their
concentration in the tumor cells. In addition, they can be made
to target the posterior segment of the eye as well, by means of functionalizing
the surface of these MNPs with VEGF to enable transcytosis into posterior
layers of the retina [17]. Once inside, they can specifically localize in the
site of interest. Nanoscale
Metal-Organic Frameworks (NMOFs) The union of organic compounds with a
nanoscale metal framework has recently been reported for the treatment of
ovarian cancer [5]. Acquired resistance to chemotherapy
is the major reason behind the dismal prognosis in ovarian cancer cases [20].
After the discovery of small interfering RNAs (siRNAs) in 1998, it is now
possible to silence certain genes. Thus, RNA interference has been shown to
undo cisplatin resistance in ovarian cancer cells [21]. A Metal Organic
Framework (MOF) is a class of self-assembling porous materials. Their
properties can be tuned to construct molecular building blocks [22]. At the
nanoscale, these NMOFs serve as nano-carriers of chemotherapeutics and imaging
contrast agents [23]. A study by Liu et
al investigates NMOFs in cancer treatment in combination with photodynamic
therapy (PDT) [24]. As PDT was approved for use in Age-Related
Macular Degeneration (AMD) about 10 years ago [25], the use of NMOFs in
treating posterior segment diseases of the eye is not far away. It is evident
that their structural and chemical properties open up far-reaching avenues in
the field of cancer targeting and ophthalmic disease treatment by making
possible the co-delivery of chemotherapeutics, such as Cisplatin, and nucleic
acids, such as siRNA, microRNA and plasmid
DNA. To summarize, targeted
drug delivery has the potential to increase therapeutic efficacy while
achieving controlled distribution as well as improving drug localization, thus
reducing drug toxicity. Gold and magnetic nanoparticles
demonstrate tremendous potential in not only treating diseases, but also
performing diagnostic testing, as well as, real-time imaging. A variety of
nanoparticle formulations show the promise of nanotechnology
in achieving targeted drug delivery (Table 1). Although significant challenges
still remain, especially in terms of reproducing similar results in clinical
trials, the studies reported thus far manifest far-reaching capabilities of nanomedicine
in cancer, ophthalmology and targeted drug delivery. 1. Kumar
Anil, Zhang Xu, L Xing-Jie. Gold nanoparticles: Emerging paradigm for targeted
drug delivery system (2013) Biotechnology Advances 593-606. 2. Mody
Vicky V, Singh Ajay, Wesley Bevins. Basics of magnetic nanoparticles for their
application in the field of magnetic fluid hyperthermia (2013) European Journal
of Nanomedicine 11-21. 3. Alexis
F, Basto P, Levy-Nissenbaum E, Radovic-Moreno AF, Zhang L, et al. HER-2-targeted
nanoparticle-affibody bioconjugates for cancer therapy (2008) Chem Med Chem 1839-1843. 4. Mody
VV, Arthur Cox, Samit Shah, Ajay Singh, Wesley Bevins, et al. Magnetic
nanoparticle drug delivery systems for targeting tumor (2014) Applied
Nanoscience 385-392. 5. Chunbai
He, Kuangda Lu, Demin Liu, Wenbin Lin. Nanoscale Metal−Organic Frameworks for
the Co-Delivery of Cisplatin and Pooled siRNAs to Enhance Therapeutic Efficacy
in Drug-Resistant Ovarian Cancer Cells (2014) Journal of the American Chemical
Society 136: 5181-5184. 6. Cunliffe
D, Kirby A, Alexander C. Molecularly imprinted drug delivery systems (2005)
Advanced Drug Delivery Reviews 57: 1836-53. 7. Cobley
CM, Chen J, Cho EC, Wang LV, Xia Y. Gold nanostructures: a class of
multifunctional materials for biomedical applications (2011) Chemical Society
Reviews 40: 44-56. 8. Schroeder
A, Heller DA, Winslow MM, Dahlman JE, Pratt GW. Treating metastatic cancer with
nanotechnology. (2012) Nature Reviews Cancer 12: 39-50. 9. Garanger
E, Boturyn D, Dum P. Tumor targeting with RGD peptide ligands-design of new
molecular conjugates for imaging and therapy of cancers (2007) Anti-Cancer
Agents in Medicinal Chemistry 7: 552-558. 10. Dreaden
EC, Megan AM, Xiaohua Huang, Bin Kanga ,
El-Sayed MA. Beating cancer in multiple ways using nanogold (2011) Chemical
Society Reviews 40: 3391-3404. 11. Montet
X, Montet-Abou K, Reynolds F, Weissleder R, Josephson L. Nanoparticle imaging
of integrins on tumor cells (2006) Neoplasia 8: 214-222. 12. Zhou
Y, Chakraborty S, Liu S. Radiolabeled cyclic rgd peptides as radiotracers for
imaging tumors and thrombosis by SPECT (2011) Theranostics 1: 58-82. 13. Song
HB, Wi JS, Jo DH, Kim JH, Lee SW. Intraocular application of gold nanodisks
optically tuned for optical coherence tomography: inhibitory effect on retinal
neovascularization without unbearable toxicity (2017) Nanomedicine:
Nanotechnology, Biology, and Medicine, 13: 1901-1911. 14. Karthikeyan
B, Kalishwaralal K, Sardarpasha S,
Venkataraman D, Ravinarayanan H. Gold
nanoparticles downregulate VEGF-and IL-1β-induced cell proliferation through
Src kinase in retinal pigment epithelial cells (2010) Experimental Eye Research
91: 769-778. 15. Kim
JH, Kim MH, Jo DH, Yu YS, Lee TG. The inhibition of retinal neovascularization
by gold nanoparticles via suppression of VEGFR-2 activation (2011) Biomaterials
32: 1865-1871. 16. Mamiya
H, Jeyadevan B. Hyperthermic effects of dissipative structures of magnetic
nanoparticles in large alternating magnetic fields (2011) Scientific Reports 1:
157. 17. Giannaccini
M, Pedicini L, De Matienzo G, Chiellini F, Dente L. Magnetic nanoparticles: a
strategy to target the choroidal layer in the posterior segment of the eye (2017)
Scientific Reports 7: 43092. 18. Keyang
Huang, Huili Ma, Juan Liu, Shuaidong Huo, Anil Kumar. Size-dependent
localization and penetration of ultrasmall gold nanoparticles in cancer cells,
multicellular spheroids, and tumors in vivo (2012) ACS Nano 6: 4483-4493. 19. Xuan
Zhang, Hicham Chibli, Randall Mielke, Jay Nadeau. Ultrasmall gold-doxorubicin
conjugates rapidly kill apoptosis-resistant cancer cells (2011) Bioconjugate
Chemistry 22: 235-243. 20. D
Roberts, J Schick, S Conway, S Biade, P B Laub. Identification of genes
associated with platinum drug sensitivity and resistance in human ovarian
cancer cells (2005) British Journal of Cancer 92: 1149–1158. 21. Yellepeddi
VK, Vangara KK, Kumar A, Palakurthi S. Comparative Evaluation of Small-molecule
Chemosensitizers in Reversal of Cisplatin Resistance in Ovarian Cancer Cells (2012)
International J Cancer Res Treatment 32: 3651-3658. 22. Hailian
Li, Mohamed Eddaoudi, M OKeeffe, OM
Yaghi. Design and synthesis of an exceptionally stable and highly porous
metal-organic framework (1999) Nature 402: 276–279. 23. Rieter
WJ, Taylor KM, An H, Lin W, Lin W. Nanoscale Metal−Organic Frameworks as
Potential Multimodal Contrast Enhancing Agents (2006) Journal of the American
Chemical Society 128: 9024–9025. 24. Jingjing
Liu, Yu Yang, Wenwen Zhu, Xuan Yi,
Ziliang Dong. Nanoscale metal−organic frameworks for combined photodynamic and
radiation therapy in cancer treatment. (2016) Biomaterials 97: 1-9. 25. Lang
GE, Mennel S, Spital G, Wachtlin J, Jurklies B. Different indications of photodynamic
therapy in ophthalmology (2009) Klin Monbl Augenheilkd 226: 725-739. Iqbal MT, Halasz K, Bhatia D (2017) Metallic Nanoparticles for Targeted
Drug Delivery. NMCT 1: 3-5 Gold Nanoparticles, Magnetic Nanoparticles (MNPs), Nanoscale Metal-Organic Frameworks (NMOFs), Metallic Nanoparticles, Targeted Drug DeliveryMetallic Nanoparticles for Targeted Drug Delivery
M Tajwar Iqbal, Kathleen Halasz and Deepak Bhatia
Abstract
Full-Text
Introduction
Gold
Nanoparticles (AuNPs)
Conclusion
and Future Perspectives
References
*Corresponding author: Deepak Bhatia,
Associate Professor of Pharmacogenomics, Shenandoah University - ICPH Fairfax,
Bernard J. Dunn School of Pharmacy, 3225 Gallows Road, Building D, Floor 3,
Fairfax, VA 22031, USA. Tel: 540-542-6239, Fax: 540-542-6280
Citation:
Keywords