Research Article :
MO Danilov, IA Rusetskii, IA Slobodyanyuk, IA Farbun and G Ya Kolbasov Catalysts for oxygen electrodes based on graphene structures
have been obtained by the chemical synthesis. Oxidizing and reducing agents
were selected on the basis of their standard redox potentials. For the obtained
materials, the morphology, physical and chemical properties, and
electrocatalytic activity for the reaction of oxygen reduction have been
investigated. It was shown that the synthesized graphene oxide and reduced
graphene oxide are promising catalyst carriers for the oxygen electrode of fuel
cells, which can replace commercial electrode materials containing platinum.
The specific capacity of the electrode based on the developed materials was
about 500 mAh g-1 at 200 mV polarization. The synthesized materials are stable
and catalytically active. The application of air or oxygen electrode in devices
generating electrical energy is useful, it does not give rise to environmental
problems and allows saving nonrenewable natural resources. Air and oxygen
electrode is a three-phase
electrode-electrolyte-gas system, where the generation of electric current
is localized
at the phase boundary. The current magnitude generated at such gas
diffusion electrode depends on the triple contact zone of these three phases.
In its turn, the electrode itself is composed of catalyst and carrier. The
interaction between them determines the quantity of generated current, which
depends on the catalyst used. It is known that nowadays the most effective
catalyst for oxygen reduction is platinum, which is a very expensive material.
A great number of works are dedicated to the investigation of other effective
but less costly catalysts [1]. Another problem is catalytically active and
stable carrier. In works the advantages of carbon
nanotubes used as the carrier are shown [2-4]. At the present time, graphene begins to be used in lithium
ion batteries and current sources, as a catalyst support for fuel cell
electrodes [5-10]. At the moment, the following methods for the synthesis of
graphene from carbon nanotubes are known: intercalation of alkaline earth
elements and nitrogen; plasma etching; microwave unzipping; unzipping with
catalytic metal nanoparticles; ultrasonic unzipping; opening-up by laser
irradiation; electrical unzipping; hydrogenation at hightemperature; unzipping
by means of a scanning
tunnel microscope; electrochemical unrolling; redox chemical synthesis
[11-29]. The chemical synthesis of graphene involves the step of
obtaining graphene oxide (GO) and its subsequent reduction to give the
so-called the reduced graphene oxide (RGO). Carbon nanotubes have a rigid
structure of graphene layers, which leads to a decrease in binding energy
between the carbon atoms in the graphene layer. Using a suitable oxidant, one
can longitudinally “unzip” nanotubes to form GO nanoribbons and then obtain RGO
by the action of a reductant. The synthesis, structure and chemical properties
of GO and RGO were systematized and described in detail in reviews [28-31]. The purpose of the work is synthesis and study of the
properties of graphene oxide and RGO, which are used as a catalyst for the
oxygen electrodes of fuel cells. We used purified reagents: H2 SO4 (98%), HF (40%), HCl
(35%), KMnO4 , NaH2 PO2 ·H2 O, H2 PtCl6 , Pb(CH3 COO)2 ·3H2 O, KOH. For the
preparation of solutions and washing we took bidistilled water. As a precursor
we used multi-walled
carbon nanotubes (MWCNT), obtained by the catalytic pyrolysis of acetylene
on a catalyst. The outer diameter of MWCNTs was about 10-30nm, with a bulk
density of 25-35gdm-3. The number of walls was 8-15. MWCNTs were purified of
catalyst by means of hydrofluoric acid treatment. Platinum is deposited by
electrochemical method from an aqueous solution containing 3%H2 PtCl6 and
0.2mass% lead acetate(II) at a voltage of 1V for 2minutes, the current
direction is changed through 30s. Based on the standard redox potentials of carbon (Table 1)
the required oxidant potential in acid medium should be more than +0.528V and
oxidant potential in alkaline medium should be more than –0.603V [32]. However,
if the process of breaking carbon bonds in nanotubes is due to kinetic
constraints, then the use of thermodynamic redox scale for this process is not
possible. Accordingly, for the reduction of graphene oxide in alkaline
medium reducing agents with potentials under –1.148V must be used. In an acidic
environment, reducing agents with potentials under –0.320V must be used to
reduce GO. As reducing agents in an alkaline medium we used solutions of sodium
hypophosphite (E○ = –1.51V) [32-33]. One gram of MWNTs was dispersed in 300ml of concentrated
sulfuric acid with stirring for one hour. Then 5g of KMnO4 was added and
stirred in an oil bath for one hour at a temperature not exceeding 17°C.
Thereafter, the mixture was heated in an oil bath to 55°C for 30minutes. Then,
the temperature was adjusted to 65°C, the mixture was allowed to stand for
20minutes and cooled to room temperature. To remove possible by-product
(manganese dioxide), the resulting mixture was poured into 400ml of bidistilled
water and ice, which contained 5ml of 30 mass% H2 O2 .
This mixture filtered using fine - pored filter paper. The filter cake was
transferred to a colloidal solution in bidistilled water. A sample of obtained
graphene oxide was dried at 140°C for three hours and used for the studies.
Another sample of oxidized product was reduced with an alkaline solution of
sodium hypophosphite (pH=11). The resulting reduced graphene oxide was filtered
using a dense, fine-pored filter paper and then separated from the filter and
dried in an oven at 140°C for three hours. The samples of RGO obtained by synthesis
were examined with the aid of a JEM-100 CXII electron microscope. The
synthesized graphene samples were examined with an electron microscope JEM-100
CXII. The X-ray phase analysis was performed with the aid of a DRON-4 X-ray
diffractometer with CuKα radiation. The porous structure of graphene materials was studied by
low-temperature nitrogen adsorption. The nitrogen adsorption/ desorption
isotherms were recorded at -196°C using a gas surface analyzer NOVA 2200
(Quantachrome, USA). The parameters of the porous structure of the samples (the
specific surface area, the volume of sorption pores, effective pore radius),
and the volume distribution of the pore size were calculated using the program
AsiQ version 3.0 for the calculations we used the BET method, the t-method, BJH
and DFT. Before the measurements, the degassing of samples at 180°C was carried
out under pressures of 1.10-4Torr for 20hours. The synthesized materials were used as the active layer of
the oxygen electrode. The hydrophobic layer contained 0.07gcm-2 acetylene black
with 25% polytetrafluoroethylene, and the active layer contained 0.02gcm-2 RGO
with 5% polytetrafluoroethylene. The investigations were carried out on a fuel
cell mockup, a zinc electrode being used as the anode. A mockup for the testing
of gasdiffusion electrodes is described in Reference [34]. The electrolyte was
a solution of 6M KOH. A silver-chloride electrode connected through a salt
bridge was used as a reference electrode. The electrochemical characteristics
were recorded under galvanostatic
conditions. The study of the polarization curves was performed on a P-8S
elins (Russia) potentiostat on a standard three-electrode system. The oxygen
source was a U-shaped electrolyzer with alkaline electrolyte. Oxygen was
supplied to the gas electrodes under an excess pressure of 0.01MPa. Before
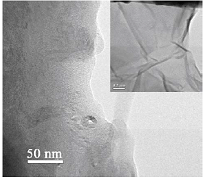
measurements, the oxygen electrode was blown through with oxygen for an hour. Figure 1 shows micrographs of GO obtained by oxidation of
MWCNTs using potassium permanganate. Figure 1 Figure 2 shows a micrograph of
RGO obtained by reduction with sodium hypophosphite the GO obtained by
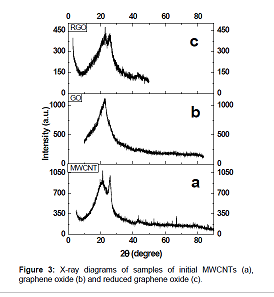
oxidation of MWCNTs using potassium permanganate. Figure 2 Figure 3 shows the
X–ray diagrams of initial multiwalled carbon nanotubes (a), graphene oxide (b)
and reduced graphene oxide (c). Figure 3 Figure 1: Micrographs of a sample of GO obtained from MWCNTs
oxidized with KMnO4 The XRD analysis showed the presence of two peaks, one of
which corresponds to the reflection from the interplanar
spacing between the graphene layers and is located at 2θ=25.6°, and the
other near 2θ=21° corresponds to SiO2 (substrate), the distance between the
planes in RGO is found to be 3.43Å, which is larger than the distance between
the planes in graphite (3.35Å). The initial carbon nanotubes and samples of
synthesized RGO have similar reflections; in the case of RGO reflection at
2θ=25.6° becomes wider, the crystallinity of the RGO sample deteriorates, which
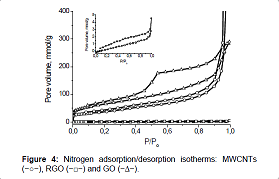
indicates a decrease in the size of particles. The nitrogen adsorption/desorption isotherms, measured for
MWCNT samples, GO and RGO, differ greatly from each other not only in form but
also in the volume of adsorbed nitrogen (Figure 4). Figure 4 For all samples,
the initial part of the isotherm, which refers to the microporous region, is
very small. For the sample of MWCNTs, presence on the isotherm of a hysteresis
loop at medium and high pressure, as well as a sharp rise in the isotherm at
the pressure P/ Po>0.9 indicate that the structure of the sample is made up
of meso and macropores. and macropores. In the nitrogen adsorption/desorption
isotherm Figure 4: Nitrogen adsorption/desorption isotherms: MWCNTs
(−○−), RGO (−□−) and GO (−∆−). for the sample of graphene oxide, an expansion of the
hysteresis loop is observed, which indicates a decrease in the number of macropores
and an increase in the proportion of mesopores in the total pore volume (type
II of IUPAC classification with H3 hysteresis loop) [34,35]. For the sample of
reduced graphene oxide, the main cause of hysteresis at low pressures
(P/Po<0.4) is activated passage of molecules into wider cavities through
existing constrictions [36]. The presence of macropores
in the structure of the MWNT sample proves the difference in the values of
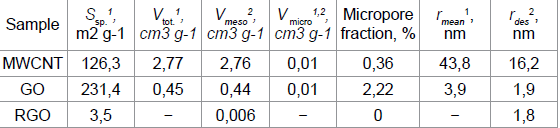
the specific surface area of this sample, obtained by the BET method (Table 1)
and the BJH method (149.2m2 g-1). Table 1 Table 1: Porosity characteristics of MWNT, RGO and GO samples Oxidation of MWCNTs to GO increases the specific surface area
by a factor of 2, reduces the pore radius and increases the number of
micropores. Since the nanopores region (0.5 to 50nm) accepted in the literature
covers micro- and mesopores, all test samples are nanoporous materials with
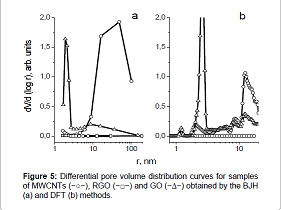
predominance of mesopores [37]. Figure 5 shows differential pore volume distribution curves,
obtained from the desorption branch of nitrogen capillary condensation using
the BJH method. Figure 5 It can be seen from Figure 5 that the most coarse –
pored material is MWCNTs with an average pore size of ≈50nm. Oxidation of
MWCNTs to GO leads to the appearance of a narrow peak (r=1.5-3nm), indicating a
narrow pore volume distribution by size. When analyzing the pore volume distribution by size by the DFT
method, was found that in the sample of MWCNTs there are mesopores with
≈10-20nm size (Figure 5b). The oxidation of MWCNTs to GO leads to the
appearance of mesopores with 1.0-1.5 and 2-3nm size. The significant reduction of specific surface area is due to
the fact that as graphene oxide reduced the hydrophilicity of its layers
gradually decreases. The graphene sheets agglomerate and the precipitate cannot
be redispersed even by ultrasonic (US) treatment [38]. To prevent
agglomeration, molecules of surfactants, biomolecules, polymers, and large
aromatic donor or acceptor molecules, which stabilize RGO by π-π interactions,
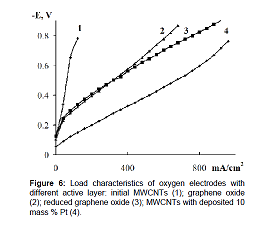
are added to the reaction mixture [39-42]. Figure 6 shows the characteristics of oxygen electrodes
based on GO (curve 2) and RGO (curve 3) and carbon nanotubes with 10 mass %
platinum deposited on them (curve 4). Figure 6 As is seen from the
current-voltage curves the electrodes based on RGO have better performance than
the electrodes based on GO. The oxygen electrodes
based on graphene oxide and reduced graphene oxide was stable for six month
under galvanostatic conditions at a current density of 200mAcm-2. For graphene
oxide doped with sulfur and nitrogen, the value of specific capacity of 415mAhg-1
at 0.9V with respect to zinc electrode is given, which corresponds to
approximately 300mV polarization. For reduced graphene oxide doped with
nitrogen with deposited LiMn2 O4 the value
of specific capacity of 585mAhg-1 at 1.15V with respect to aluminum electrode
is given, which corresponds to approximately 400mV polarization [43,44]. The
specific capacity calculated for our electrodes at 200mV polarization is about
500mAhg-1. Thus, the proposed simplified procedure for obtaining samples of
oxidized graphene and reduced graphene oxide can produce materials for catalyst
carriers which are not inferior to the existing analogues. Conclusions The synthesized graphene oxide and reduced
graphene oxide promise much as catalyst carriers for the oxygen electrode
of fuel cell, which can replace the commercial electrode materials containing
platinum. 1. Bidault F, Brett DJL, Middleton PH, et al. Review of gas
diffusion cathodes for alkaline fuel cells (2009) J Power Sourc 187: 39–48. 2. Soehn M, Lebert M, Wirth T, et al. Design of gas
diffusion electrodes using nanocarbon (2008) Ibid 176: 494–498. 3. Hsieh CT, Lin JYi, Wei JL. Deposition and electrochemical
activity of Ptbased bimetallic nanocatalysts on carbon nanotube electrodes
(2009) Int J Hydrogen Energy 34: 685–693. 4. Wang X, Waje M, Yan Y. CNT-Based Electrodes with High
Efficiency for PEMFCs (2005) Electrochem Solid-State Lett 8: A42–A44. 5. Wang G, Shen X, Yao J, et al. Graphene nanosheets for
enhanced lithium storage in lithium ion batteries (2009) Carbon 8: 2049–2053. 6. Xin Y, Liu J, Jie X, et al. Preparation and
electrochemical characterization of nitrogen doped graphene by microwave as
supporting materials for fuel cell catalysts (2012) Electrochim Acta 60:
354–358. 7. Lin Z, Waller G, Liu Y, et al. Facile synthesis of
nitrogen-doped graphene via pyrolysis of graphene oxide and urea and its
electrocatalytic activity toward oxygen reduction reaction (2012) Adv Energy
Mater 2: 884–888. 8. Qu L, Liu Y, Baek JB, et al. Nitrogen-doped graphene as
efficient metalfree electrocatalyst for oxygen reduction in fuel cells (2010)
ACS Nano 4: 1321-1326. 9. Lin Z, Song MK, Ding Y, et al. Facile preparation of
nitrogen-doped graphene as a metal-free catalyst for oxygen reduction reaction
(2012) Phys Chem Chem Phys 14: 3381-3387. 10. Shao Y, Zhang S, Wang C, et al. Highly durable graphene
nanoplatelets supported Pt nanocatalysts for oxygen reduction (2010) J Power
195: 4600–4605. 11. Cano Márquez AG, Rodriguez Macias FJ, Campos Delgado J,
et al. ExMWNTs: Graphene sheets and ribbons produced by lithium intercalation
and exfoliation of carbon nanotubes (2009) Nano Lett 9: 1527–1533. 12. Kosynkin DV, Lu W, Sinitskii A, et al. Highly conductive
graphene nanoribbons by longitudinal splitting of carbon nanotubes using
potassium vapor (2011) ACS Nano 5: 968-974. 13. Morelos-Gómez A, Vega-Díaz SM, González VJ, et al. Clean
nanotube unzipping by abrupt thermal expansion of molecular nitrogen: graphene
nanoribbons with atomically smooth edges (2012) Ibid 6: 2261–2272. 14. Jiao L, Zhang L, Wang X, et al. Narrow graphene
nanoribbons from carbon nanotubes (2009) Nature 458: 877-880. 15. Valentini L. Formation of unzipped carbon nanotubes by
CF4 plasma treatment (2011) Diamond & Related Materials 20: 445–448. 16. Mohammadi S, Kolahdouz Z, Darbari S, et al. Graphene
formation by unzipping carbon nanotubes using a sequential plasma assisted
processing (2013) Carbo 52: 451–463. 17. Janowska I, Ersen O, Jacob T, et al. Catalytic unzipping
of carbon nanotubes to few–layer graphene sheets under microwaves irradiation
(2009) Appl Catal V 371: 22–30. 18. Vadahanambi S, Jung J–H, Kumar R. et al. An
ionic liquid-assisted method for splitting carbon nanotubes to produce graphene
nano–ribbons by microwave radiation (2013) Carbon 53: 391–398 19. Elías A.L, Botello-Méndez As.R, Meneses-Rodríguez D. et
al. Longitudinal cutting of pure and doped carbon nanotubes to form graphitic
nanoribbons using metal clusters as nanoscalpels (2009) Nano Lett 10: 366–372 20. Parashar UK1, Bhandari S, Srivastava RK, Jariwala D,
Srivastava A. Single step synthesis of graphene nanoribbons by catalyst
particle size dependent cutting of multiwalled carbon nanotubes (2011)
Nanoscale 3: 3876-3882. 21. Jiao L1, Wang X, Diankov G, Wang H, Dai H. Facile
synthesis of highquality graphene nanoribbons (2010) Nat Nanotechnol 5:
321-325. 22. Xie L1, Wang H, Jin C, Wang X, Jiao L, et al. Graphene
nanoribbons from unzipped carbon nanotubes: atomic structures, Raman
spectroscopy, and electrical properties (2011) J Am Chem Soc 133: 10394-10397. 23. Kumar P1, Panchakarla LS, Rao CN. Laser-induced
unzipping of carbon nanotubes to yield graphene nanoribbons (2011) Nanoscale 3:
2127-2129. 24. Kim K1, Sussman A, Zettl A. Graphene nanoribbons
obtained by electrically unwrapping carbon nanotubes (2010) ACS Nano 4: 1362-1366.
25. Talyzin AV1, Luzan S, Anoshkin IV, Nasibulin AG, Jiang
H, et al. Hydrogenation, purification, and unzipping of carbon nanotubes by
reaction with molecular hydrogen: road to graphane nanoribbons (2011) ACS Nano
5: 5132-5140. 26. Paiva MC1, Xu W, Proença MF, Novais RM, Laegsgaard E, et
al. Unzipping of functionalized multiwall carbon nanotubes induced by STM.
(2010)Nano Lett 10: 1764-1768. 27. Shinde DB1, Debgupta J, Kushwaha A, Aslam M, Pillai VK.
Electrochemical unzipping of multi-walled carbon nanotubes for facile synthesis
of highquality graphene nanoribbons (2011) J Am Chem Soc 133: 4168-4171. 28. Kosynkin DV1, Higginbotham AL, Sinitskii A, Lomeda JR,
Dimiev A, et al. Longitudinal unzipping of carbon nanotubes to form graphene
nanoribbons (2009) Nature 458: 872-876. 29. Zhang S., Zhu L., Song H. et al. How graphene is
exfoliated from graphitic materials: synergistic effect of oxidation and
intercalation processes in open, semi-closed, and closed carbon systems (2012)
J. Mater. Chem 22: 22150–22154 30. Zhu Y1, Murali S, Cai W, Li X, Suk JW, et al. (2010)
Graphene and graphene oxide: synthesis, properties, and applications. See
comment in PubMed Commons below Adv Mater 22: 3906-3924. 31. Pei S, Cheng H.M. The reduction of graphene oxide (2012)
Carbon 50: 3210–3228 32. Bratsch S.G. Standard electrode potentials and
temperature coefficients in water at 298.15 (1989) J. Phys. Chem 18: 1–21 33. Danilov M.O, Kolbasov G.Ya, Rusetskii I.A. et al.
Electrocatalytic properties of multiwalled carbon nanotubes-based
nanocomposites for oxygen electrodes (2012) Russian J. Appl. Chem 85: 1536–1540
34. Gregg S.J, Sing K.S.W. Adsorption, surface area and
porosity (1982) London: Academic Press 310 35. Sing K.S.W, Everett D.H, Haul R.A.W. et al. Reporting
physisorption data for gas/solid systems with special reference to the
determination of surface area and porosity (1985) Pure Appl. Chem 57: 603–619 36. Zhang P, Xu F, Navrotsky A. et al. Surface enthalpies of
nanophase ZnO with different morphologies (2007) Chem 19: 5687-5693 37. Edelstein A.S., Cammarata K.C. Nanomaterials: synthesis,
properties and applications (1998) Washington: CRC Press 616 38. Stankovich S, Dikin D.A, Piner R.D, et al. Synthesis of
graphene-based nanosheets via chemical reduction of exfoliated graphite oxide
(2007) Carbon 45: 1558-1565 39. Stankovich S1, Dikin DA, Dommett GH, Kohlhaas KM, Zimney
EJ, et al. (2006) Graphene-based composite materials. See comment in PubMed
Commons below Nature 442: 282-286. 40. Patil A.J, Vickery J.L, Scott Th. B Mann S. Aqueous
stabilization and selfassembly of graphene sheets into layered
bio-nanocomposites using DNA (2009) Adv. Mater 21: 3159-3164 41. Xu Y, Bai H, Lu G, et al. Flexible graphene films via
the filtration of watersoluble noncovalent functionalized graphene sheets
(2008) J. Am. Chem. Soc 130: 5856-5857 42. Liu H, Gao J, Xue M, et al. Processing of graphene for
electrochemical application: noncovalently functionalize graphene sheets with
water-soluble electroactive methylene green (2009) Langmuir 25: 12006-12010 43. Ganesan P, Ramakrishnan P, Prabu M, Shanmugam S.
Nitrogen and Sulfur Co-doped Graphene Supported Cobalt Sulfide Nanoparticles as
an Efficient Air Cathode for Zinc-air Battery (2015) Electrochim. Acta 183:
63-69 44. Liu Y, Li J, Li W, et al. Spinel LiMn2O4 nanoparticles
dispersed on nitrogendoped reduced graphene oxide nanosheets as an efficient
electrocatalyst for aluminium-air battery (2015) International Journal of
Hydrogen 40: 9225- 9234. Danilov MO, Rusetskii IA, Slobodyanyuk, IA Farbun, Kolbasov
G Ya (2015) Synthesis and Electrocatalytic Properties of Materials Based On
Graphene Structures. NMCT 104:1-5Synthesis and Electrocatalytic Properties of Materials Based On Graphene Structures
Abstract
Full-Text
Introduction
Experimental
Results and Discussion

References
*Corresponding author: Rusetskii IA, Vernadskii Institute of
General & Inorganic Chemistry of Nat Acad Sci Ukraine, Prospekt Palladina
32-34, 03680 Kyiv 142, Ukraine, Tel: +380 44 4242280 E-mail: rusetskii@ionc.kiev.ua
Citation:
Keywords