Review Article :
Prostate cancer is a major public health
concern, particularly in the welfare countries, for this reason, screening
should be considered to reduce the number of deaths. Screening tests are
available, i.e. digital rectal examination; trans-rectal ultrasonography and
prostate specific antigen, nevertheless their sensitivity, specificity and
positive predictive value are far from being perfect. Evidences from randomized
screening trials are still indebted for conclusive evidence. The screening
might cause more harm than good due to over diagnosis and over-treatment as a
result of limited specificity of the screening tests. According to our point a
view, opportunistic screening as part of diagnostics of patients having
suspicion for uncertain symptoms of prostatic disorder is fully justified but
mass screening of the population of average risk should not be introduced until
supportive evidence from randomized controlled trials would be available. Prostate cancer is one of the most controversial issues of medical
practice, preventive oncology and health care system. Some specialists argue
for the necessity of screening, recommend all men aged 50 years and older to
regularly undergo screening examination, and the initiative is welcomed by the
public at large. Others are reluctant to put the prostate screening on the
agenda of public health. The aim of this paper is to overview the currently
available evidences and arguments, and tries to find answer to the question: to
screen or not to screen. According to the World Cancer Report 2014, prostate cancer is the
second most frequently diagnosed cancer in men [1]. In 2016, there were 1.4
million incident cases of prostate cancer and 381.000 deaths worldwide.
Prostate cancer is one of the leading causes of mortality in the global
mortality statistics, such as GLOBOCAN database [2], however the relatively
high mortality rates might be virtual; in case of those who have been diagnosed
with prostate cancer during their lifetime, the death certificate would
indicate prostate cancer as cause of death, let the real cause of death be any
other inter-current disease. There are tremendous geographical differences
in the incidence among the various regions of the Globe. In the economically
developed welfare countries such as the Unites States and Nordic and Western
countries of Europe, prostate cancer is a major public health problem, and a
major burden on the societies, the patients and their relatives. The rates are
several times higher in more developed countries compared with less developed
ones. A positive correlation was observed between the standardized
incidence rates of prostate cancer and the Human Development Index (HDI), components
of which are life expectancy at birth, mean years of schooling, and the gross
national income per capita. In addition, there was a negative correlation
between standardized mortality rates and HDI [3,4]. The increasing incidence
together with an aging and growing population have led to a more than 3-fold
increase in prostate cancer cases since 1990. In the same time, the death rates
for prostate cancer seems to decrease in the majority of more developed
countries attributed mainly to improved treatment and early detection efforts.
Prostate cancer incidence rates are still lower in developing countries than in
developed countries, but because of a faster increase in rates in developing
countries, the gap decreased between 1990 and 2013 from a 4-fold to a 3-fold
difference [5]. Prior to Prostate Specific Antigen (PSA) testing it was rare to
diagnose before the age 55 years. Since introduction of PSA-test there has been
a dramatic increase in the number of men diagnosed with prostate cancer in
their late 50s and early 60s [6]. Risk factors for prostate cancer are numerous [7]. The greatest
risk factor for prostate cancer is age: this risk increases after the 50 years
of age; the majority are diagnosed in men age 65 years of age and older. Family
history might have a role to play: men whose relatives have had prostate cancer
are considered to be at higher risk; the hereditary form of prostate cancer
accounts for just 5% to 10% of all cases. Lifestyle-related factors, such as
physical activities, smoking and alcohol consumption are not closely linked to
prostate cancer. High dietary fat may be a contributing factor for prostate
cancer. Several studies have examined the relationship between prostate cancer
and antioxidants; however, the results of these studies are inconsistent [8].
Studies show higher incidence among migrants moving from low- to high-risk area
[9]. The role of hormone-profile is verified (androgens,
androgen-receptors, insulin-like growth) [10]. Prostate cancer has a long
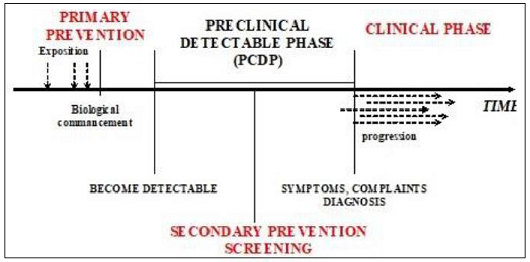
natural history, and a long Pre Clinical Period (PCDP) during which it can be
detected (Figure 1). Figure 1:
Natural history of disease development. Cancer of prostate manifests itself in a various ways. Clinical
carcinoma includes those cases in which diagnosis of the prostate cancer is
made clinically and confirmed histologically. Occult carcinoma is manifested by
its metastases before the primary site is detected. Subclinical carcinoma
comprises incidental carcinoma which is discovered on microscopic examination
of prostate tissue removed surgically for non-malignant disease, and latent
carcinoma which is found at autopsy in patient who had had no clinical evidence
of prostatic cancer [11]. Most prostate cancers are classified as
adenocarcinoma. The primary tumours begin when normal epithelial gland cells
mutate into cancer cells. The intraepithelial neoplasia locates in the
peripheral zone of the gland, and atypical adenomatous hyperplasia in the
transitional zone; these conditions are considered precursors or preclinical
conditions, which can be detected by suitable methods. It is very often
multi-focal. There is no real evidence for a relationship between Benign
Prostatic Hyperplasia (BPH) and cancer [12]. It may spread locally to
periprostatic structures, pelvic lymph nodes, and then through the bloodstream
to bones, lungs and liver. The rate of progression, thus the prognosis
correlates with the degree of cellular differentiation, as reflected in the
Gleasons system of grading [13,14]. For early detection of prostate disorders, for the time being,
three methods are at our disposal: Rectal Digital Examination (RDE), Trans
Rectal Ultrasonography (TRUS), and measurement of Prostate-Specific Antigen in
the serum (PSA). Digital rectal examination is the oldest invasive
test. As early as 1905, Hugh Hampton Young suggested that a careful
rectal examination could identify changes in prostate gland that could lead to
early diagnosis of cancer and early treatment [15]. The sensitivity of rectal
examination is limited because its range is no longer than that of the pointing
finger of examiner, consequently, it can only palpate rear and lateral surfaces
of the gland, but it cannot reach the front and middle surfaces, and the smaller
nodules within the gland either. The specificity is also less than optimal
because the majority of positive findings corresponds to Benign Prostate
Hyperplasia (BPH), so too many false positive findings occur. The positive
predicting value (PPV) of is very low (25-40%) [16]. Given, the considerable
lack of evidence supporting its efficacy, the available literature recommend
against routine performance of DRE to screen for prostate cancer [17]. However,
it should remain a part of routine medical examination. Transrectal ultrasonography provides more information about the
prostate and its environment. The capsule of the gland, seminal vesicle and
bladder neck can be visualized. It can detect nodules of 5mm in diameter. It
was first developed in the 1970s. TRUS-guided biopsy, under local anaesthetic
and prophylactic antibiotics, is now the most widely accepted method to
diagnose of prostate cancer [18]. However, the interpretation requires
considerable expertise; the test is quite expensive and difficult to perform.
The sensitivity and specificity of TRUS in the detection of prostate cancer is
low, so it has not been accepted as screening tool. TRUS is useful in guiding
fine-needle aspiration. Prostate-specific antigen is a glycoprotein produced only by the
epithelial cells of prostatic origin. Being present in both benign and
malignant epithelial cells, prostate-specific antigen can be detected and
quantified in the serum. Those with prostate cancer may have serum PSA level
higher than the normal ones. However, it is not specific to carcinoma of
prostate, and up to the present, such threshold value could not be established
which would definitively indicate the presence of prostate cancer [19]. On
the other end, Catalona et al in 1991 published a study in which PAS level was
elevated in 25% of prostate cancer cases [20]. The elevated concentration of
PSA in the serum is not exclusively characteristic to prostate cancer but a
number of other conditions such as inflammation, benign prostate hypertrophy;
even ejaculation and digital rectal examination can cause elevated PSA level
[21]. Nevertheless, today it is generally accepted that PSA detection in serum
is the most sensitive, most appropriate method of detection of prostate cancer. The cut-of point between the benign and malignant samples needs to
be clearly defined. The normal range for PSA has been established as less than
4ng/ml. A level over 10ng/mm is most unlikely to be due to prostatitis or
benign prostatic hyperplasia, and it most likely indicates prostatic cancer,
therefore urologic examination is suggested. The borderline values (4-9.9
ng/ml) need to be interpreted in light of clinical findings. Minimally elevated
PSA values need to be repeated before considering prostate ultrasound and
biopsy. A positive PSA-test is not more than expression of suspicion for
prostate cancer; therefore, it needs to be histologically confirmed. Though the
histopathology should be regarded as golden standard of diagnostics of prostate
cancer, it is difficult to judge the accuracy of the procedure because the
biopsy itself - as a result of the uncertainty of sampling - carries a debt
with detection of 10-30% of cancer cases. The sensitivity of PSA-test in the
detection of prostate cancer is not more than 20% and only 50% in the advanced
cancer. The positive predictive value for a PSA level >4.0ng/ml is
approximately 30 percent, meaning that slightly less than one in three men with
an elevated PSA will have prostate cancer detected by biopsy [22]. The impact
of prostate-specific antigen testing on prostate-cancer mortality. PSA
screening for prostate cancer is highly controversial. There is considerable
controversy regarding the benefits, and risks of population-based screening for
prostate cancer. Evaluating the effect of prostate-specific antigen on the impact
of prostate-cancer mortality, up to now, two Randomized Controlled Trials (RCT)
were published: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer
Screening Trial, conducted by the National Cancer Institute [23] and the
European Randomized Study of Screening for Prostate Cancer (ERSPC) supported by
Europe against Cancer, and multiple European agencies and health authorities
[24]. Several poor-quality randomized trials and cohort studies have been
excluded from the review. The strength of PLCO trial was a common protocol
by ten study centers in the United States. On the other hand, the lack of
common design and protocol makes the ERSPC study the more difficult to
interpret; it was a collection of seven PSA screening trials employing
different study design, screening tests, screening intervals, and different
ages of patients at entry and choices of controls. In both studies,
in the control groups, they had made efforts to minimalise the most common
contaminating factor: namely, the opportunistic screening tests. During 1995
the ERSPC and PLCO study groups explored the possibility of co-operation,
specifically the option of a common analysis, as opposed to a meta-analysis, of
both trials. Conditions for a common analysis were defined and described in
1996 [25]. The PLCO trial was initiated in 1993, and randomized 76,683 men
between 55-74 years of age to an experimental group, and 38.343 subjects as control
group. The screening test was annual PSA over 6 years, completed with digital
rectal examination. The control group has not been examined PSA, however, some
half of the participants had been contaminated with PSA test outside the trial.
Considering all PLCO participants, prostate cancer was diagnosed in 4250 men in
the screened group and in 3815 men in control group. There was no significant
difference in prostate cancer mortality after 13 years of follow-up. Prostate
cancer survival was extremely high in both groups. Only 303 of 8065 prostate
cancer patients (3.75%) died of prostate cancer after 13 yrs. They
have concluded that while PLCO revealed no differences in prostate cancer
mortality or survival, the high degree of PSA contamination in control group
undoubtedly led to the dramatically favourable outcomes among PLCO participants
[26,27]. The European Randomised Study of Screening for Prostate Cancer
(ERSPC) was initiated in 1993 to evaluate the effect of screening with
Prostate-Specific-Antigen (PSA) testing on death rates from prostate cancer.
182,000 men between the ages of 50 and 74 years were identified through
registries in seven European countries for inclusion in the study. Each study
had different recruitment and randomization practices. The men were randomly
assigned to a group that was offered PSA screening at an average of once every
4 years or to a control group that did not receive such screening. 82% of men
accepted at least one offer of screening. During a median follow-up
of 9 years, the cumulative incidence of prostate cancer was 8.2% in the
screening group and 4.8% in the control group. The absolute risk difference was
0.71 deaths per 1000 men. PSA-based screening reduced the rate of death from
prostate cancer by 20% but was associated with a high risk of overdiagnosis
[28]. Analyses after 2 and 4 additional years of follow-up consolidated the
previous findings. In this update the ERSPC confirms a substantial reduction in
prostate cancer mortality attributable to testing of PSA, with a substantially
increased absolute effect at 13 years compared with findings after 9 and 11
years. Despite our findings, further quantification of harms and their
reduction are still considered a prerequisite for the introduction of
populated-based screening [29,30]. The ERSPC also investigated how often PSA should be tested. It has
been found that in 90% of men, whose PSA value in the first
screening round was 1.9ng/ml, 4 years later, in the second round, the PSA value
increased to 3ng/ml (at a cutoff point 4ng/ml). From this, they concluded that
annual PSA screening is meaningless [31]. The final conclusion of ERSPC is
that PSA-based screening significantly reduced mortality from prostate cancer
but did not affect all-cause mortality. Unfortunately, the PSA test results in
too much over diagnosis. As a result, the healthcare system is overloaded with
too many over treated cases, and there are too many avoidable psychological
side effects [33]. The solution could be the use of new, more specific biomarkers
[33,34]. One can conclude that as per today measurement of prostate
specific antigen is the first-line method for screening for prostate cancer.
PSA-test may or may not be completed by Digital Rectal Examination (DRE).
However, the sensitivity and specificity of the test are far from being
optimal. Before a screening programme can be introduced, it must satisfy
the requirements that it does more good than harm. No question, the measurement
of prostate specific antigen as screening test may help to detect prostate
cancer at an earlier stage, but it does not have a significant impact on either
overall mortality or death from prostate cancer. ERSPC showed a 20% reduction
in prostate cancer mortality after 13 years of follow-up, PLCO showed a
non-significant benefit in favor of the control. A meta-analysis by Cochrane
review of both trials suggested that screening does not significantly affect
prostate cancer mortality [35]. Observational data shows a considerable
increase in untreated prostate cancer mortality only 25 years after diagnosis,
suggesting that a possible effect of screening would take a comparable time
span to become apparent [36]. However, the benefit-to-harm ratio remains uncertain. Because of
limited specificity, PSA screening is associated with false-positive results,
over diagnosis and overtreatment, including biopsy complications. Over
diagnosis is very common. In a meta-analysis of sixty-three studies in 104
publications including 1 904 950 men, over diagnosis was estimated to occur in
20.7% to 50.4% of screen-detected cancers [37]. Due to limited specificity of PSA test, false-positive results,
over diagnosis and the consequent overtreatment present a more pressing
problem. The screening might bring a number of test-positive cases to the
surface which without screening would have been symptomless until the end of
their life. As Richard M. Ablin, inventor of the PSA says about the great
prostate hoax: Every year, more than a million men undergo painful needle
biopsies for prostate cancer, and upward of 100,000 have radical
prostatectomies, resulting in incontinence and impotency. But the shocking fact
is that most of these men would never have died from this common form of
cancer, which frequently grows so slowly that it never even leaves the prostate
[38]. The treatment of these cases is unnecessary; it adversely affects the
quality of life and may result in avoidable complications [39,40]. Because
treatment has potential side effects, it is critical that not all patients with
prostate cancer receive aggressive treatment. Psychological adverse effects need to be mentioned [41,42].
Screening may adversely affect quality of life not only by inducing over
diagnosis and overtreatment. Anxiety goes along with waiting for the results,
and especially with interpreting them. Three out of four elevated PSA levels
were not caused by prostate cancer. That does not only mean that three quarters
of men undergoing biopsy are confronted with a false-positive result, and means
the start of a long phase of uncertainty and fear of disease. Especially, when
an elevated PSA level is not the result of a measuring error, but persists or
even increases over time. With a 5- or 10-year history of slowly but
continuously increasing PSA levels, uncertainty may grow to such an extent that
a prostate cancer diagnosis may come as a relief. Without screening, they would
have had up to 10 more years in which they could have felt healthy without
worsening their prognosis. Prostate cancer is a major health problem worldwide, particularly
in the developed, welfare countries, but it is a growing problem in the less
developed countries, too. Most of the medical profession argues for making the
benefits of screening available for the public at large. On the other side, the
public health profession is reluctant to promote the introduction of prostate
screening into the healthcare system. Several trials are published
evaluating the effect of prostate screening have shown conflicting results. The
European trial (ERSPC) showed a 20% reduction in prostate cancer mortality
after 13 years of follow-up. On the other hand, the American trial (PLCO)
showed a non-significant benefit in favor of the control. A meta-analysis of
both trials suggested that screening does not significantly affect prostate
cancer mortality. The question arises whether screening for prostate cancer does
make any sense, or not? Screening refers to repeated testing of
asymptomatic persons applying simple, safe, relatively inexpensive, sensitive
and specific methods, suitable for detection of the target condition earlier
when it would have been clinically manifested. The overall aim of screening is
to favorable modify the course of disease development, to treat it in an early,
preclinical stage, when the disease is more responsible to curative treatment,
thereby preventing clinically manifest, advanced disease, and fatal
outcome. There are two levels on which screening decisions are made:
public health level making health policy decisions followed by implementation
of call-and-recall system of screening, based on individual identification of
the persons at risk by age in the population, and the individual level: every
man making his personal decision whether or not to undergo a screening test.
Since the principles of Evidence-Based Medicine (EBM) have been applied in the
public health area, there are strict criteria for initiating organized
population-based screening in the healthcare system: mortality rates from the target
disease are expected to significantly decrease in the target population,
attributable to the screening efforts. The evidence can only be obtained from
randomized controlled trials (RCT): only reduced mortality in a randomized
trial constitutes evidence of the benefit of screening [42]. Screening
programmes should be undertaken only when their effectiveness has been
demonstrated. All the rest of evidences are biased, because the slowly growing
tumours are more likely to stick to the screen than the fast growing ones,
therefore are represented in a larger number (length bias), or the diagnosis is
advanced in time, thus survival gain is only illusory (lead bias) [43]. In
the healthcare system, screening can be applied in two different ways: opportunistically
and in an organized manner. Opportunistic screening is a component of
medical/urological practice offering the test whenever opportunity arises,
while organized or population screening is a public health measure performed by
individual identification, personal invitation of each person on high risk by
age [44]. Most of the national and international professional societies
published their recommendation for prostate screening. The advice to implement
the currently available knowledge is summarized in guidelines. The American
Cancer Society, the American Association of Urologists, and the American
Society of Family Physicians, the American Society of Clinical Oncologist
recommend that each men over 50 years of age who have a life expectancy more than
10 years should be advised to annually undergo prostate screening with prostate
specific antigen test wit/without digital rectal examination. The US
Preventive Services Task Force [45], the official forum of disease prevention
in the USA recommended against PSA screening in all men saying that there was
insufficient evidence advising the public either for or against it. However,
more benefit can be expected from screening of men of 55-69 years of age; over
75 years of age the screening is meaningless therefore not recommended at all.
As to the age-range to be screened is concerned, the PSA test loses its
significance over time because its long natural history the prostate cancer is
slowly developing over the years, therefore it is more likely to die from some
inter-current disease than from prostate cancer. The US Preventive
Services Task Force (USPSTF) emphasized that prostate cancer should be an
individual one and should include informed decision, i.e. discussion of the
potential benefits and harms of screening with their patients [46]. Screening
offers a small potential benefit of reducing the chance of death from prostate
cancer in some men. However, many men will experience potential harms of
screening, including false-positive results that require additional testing and
possible prostate biopsy, over diagnosis and overtreatment; and treatment
complications. In determining whether this service is appropriate in individual
cases, patients and clinicians should consider the balance of benefits and
harms on the basis of family history, race/ethnicity, comorbid medical
conditions, patient values about the benefits and harms of screening and
treatment-specific outcomes, and other health needs. Clinicians should not
screen men who do not express a preference for screening. The USPSTF recommends
against PSA-based screening for prostate cancer in men 70 years and older. The
American Society of Clinical Oncology followed by agreeing with this approach
only for older men but advising informed decision-making in younger men [47]. These recommendations have been adopted by both Canadian and
European professional societies [48,49]. Guidelines on prostate cancers
screening summarize the most recent findings and advice for the use in the
medical practice. In Northern and Western Europe, the number of men diagnosed
with prostate cancer has been on the rise. This may be due to an increase in
opportunistic screening, but other factors may also be involved (eg, diet,
sexual behavior, etc.). The 2013 update of the European Association of
Urologists Guidelines on Prostate Cancer recommends screening on an individual
basis which also implies a baseline measurement of PSA in all men aged 40-45
years to initiate a risk-adapted follow-up approach. The goal should therefore
be to maximize the benefits of PSA testing for prostate cancer screening and
minimize its harms [50]. Until now, in the public health field, we do not
have the kind of convincing evidence, which could provide a solid basis for a
decision to introduce organized population-based mass screening for prostate
cancer. In the same time, the ongoing opportunistic screening is considered
controversial due to the considerable risk of detecting latent cancers. As a result of the widespread opportunistic screening, much more
prostate cancer come to light and treated than it would have been without
screening, but mortality does not decrease in the same proportion. The decline
in mortality, however, has a huge price. The number of men needed to treat to
prevent one death is rather high in prostate cancer screening. According to the
ERSPC trial, 1055 screening need to perform to prevent one death; meanwhile 192
false positive results, 37 over diagnosis and overtreatment, 12 cases of
impotence and 4 cases of urinary dysfunction may occur [51]. To the
question that should mass screening for prostate cancer be introduced at
national level, the Health Evidence Network program of World Health
Organization (WHO) European Regional Office the aim of was the evaluation of
the effectiveness of healthcare technologies stated that Studies in different
populations do not provide good evidence that mass screening for prostate
cancer does more good than harm [52]. In Europe, the Advisory Committee on
Cancer Prevention explicitly stated that until we have convincing evidence that
the PAS test reduces mortality in the target populations, and has a positive
impact on quality of life, a screening program for detection of prostate cancer
as a public health procedure cannot be recommended [53]. The organized population screening for cancer is defined as
personal invitation of asymptomatic persons at risk by age, and their
examination by suitable method in order to determine whether a particular
target disease is likely to be established or likely to be excluded.
Precondition of introduction of such a screening program is that in randomized
controlled trials, after a reasonable period of time the mortality of the
target disease decreases in the target population, attributable to the
screening. For the time being, there are no evidences available of the
effectiveness of screening for cancer of prostate in terms of mortality
reduction, therefore – according to the state-of-the art organized population
screening for prostate cancer is not justifiable. However, prostate
specific antigen is an indispensable screening tool, which is recommended in
those subjects who are clinically suspected of any prostate abnormalities, or,
for some reason are classified as high-risk persons for prostate cancer. PSA-test
can be performed by urologist or even family physicians on the basis of their
medical judgment and oncology alertness, having the informed consent of the
patient. It must be kept in mind that due to limited specificity and
sensitivity of prostate specific antigen is limited, and it can manifest itself
in a high degree of over diagnosis, and this might lead to unavoidable
psychological side effects and an avoidable burden on the healthcare
system.Regarding to the lack of prospects for primary prevention, the high
incidence and relatively high mortality of prostate cancer, introduction of the
large scale screening means a serious dilemma to be solved. It would be
desirable to exploit the blessings of secondary prevention for sake of the
populations. The long natural history of the disease would make it possible.
Unfortunately, at present this is not feasible, the screening test can be
applied only on an individual basis, in consultation with a physician [54].
Until the ongoing studies provide evidence of the effectiveness of organized
population screening, we have to apply the screening test with self-restraint. 1. https://www.drugsandalcohol.ie/28525/1/World%20Cancer%20Report.pdf 2. Wong MCS, Goggins WB, Wang HH, Fung FD, Leung C,
et al. Global incidence and mortality for prostate cancer: analysis of temporal
patterns and trends in 36 countries (2016) Eur Urol 70: 862-874. 3. Hassanipour-Azgomi S, Mohammadian-Hafshejani A,
Ghoncheh M, Towhidi F, Jamehshorani S, et al. Incidence and mortality of
prostate cancer and their relationship with the Human Development Index
worldwide (2016) Prostate Int 4:118-124. 4. Fidler MM, Soerjomataram I, Bray F. A global
view on cancer incidence and national levels of the human development index
(2016) Int J Cancer 139:2436-2446. 5. Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam
T, Alizadeh-Navaei R, et al. Global Burden of Disease Cancer Collaboration.
Global, regional, and national cancer incidence, mortality, years of life lost,
years lived with disability, and disability-adjusted life-years for 29 cancer
groups, 1990 to 2016: A systematic analysis for the global burden of disease
study (2018) J Amer Med Assoc Oncol 4:1553-1568. 6. Welch AG, Albertsen PC. Prostate cancer
diagnosis and tratment after the introduction of prostate specisic antigen
screening: 1985-2003 (2009) J Natl Cancer Inst 101:1325-1329. 7. Patel AR, Klein EA. Risk factors for prostate cancer
(2009) Nat Clin Practice Urol 6:87–95. 8. Vance TM, Su J, Fontham ET, Koo SI and and Chun
OK. Dietary antioxidants and prostate cancer: a review (2013) Nutr Cancer
65:793-801. 9. Dearnaley DP. Cancer of the prostate (1994) Brit
Med J 308:780-784. 10. Gann PG. Risk Factors for Prostate Cancer (2002) Rev Urol 4:53-60. 11. Döbrőssy L. Prevention in primary care: Recommendations for
promoting good practice (1994) WHO, Copenhagen, Denmark. 12. Roehrborn CG. Benign Prostatic Hyperplasia: an overview (2005) Rev
Urol 7:3-14. 13. Chen N, Zhou O. The evolving Gleason grading system (2016) Chin J
Cancer Res. 28:5-64. 14. Samaratunga H, Delahunt B, Egevad L, Srigley JR
and Yaxley J. The evolution of Gleason grading of prostate cancer
(2017) J Diagn Pathol 12:5-11. 15. Young HH. Early diagnosis and radical cure of carcinoma of the
prostate gland (2002) Bull John Hopkins Hosp 168:914-421. 16. Jones D, Friend Ch, Dreher A, Allgar V and Macleod U. The diagnostic
test accuracy of rectal examination for prostate cancer diagnosis in
symptomatic patients: a systematic review (2018) BMC Family Practice 19:79-84. 17. Naji L, Randhawna H, Sohani Z, Dennis B, Lautenbach D,
et al. Digital Rectal Examination for prostate cancer screening in primary
care: a systematic review and meta-analysis (2018) Ann Fam Med 16:149-154. 18. Harvey CJ, Pilcher J, Richenberg J, Patel U and Frauscher F.
Applications of trans-rectal ultrasound in prostate cancer (2012) Br J Radiol
85:3-17. 19. Haythorn MR, Ablin MR. Prostate-specific antigen testing across in
serum across the spectrum of prostate cancer (2011) Biomarker Med 5:515-526. 20. Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, et al.
Measurement of prostate-specific antigen in serum as a screening test for
prostate cancer (1991) N Engl J Med 324:1156-1161. 21. Lin K, Lipsitz R and Miller TH. Benefit and harms of PSA screening
for prostate cancer: An evidence update for the USPSTF (2008) Ann Int Med
149:192-199. 22. Schröder FH, van der Cruijsen-Koeter I, de Koning HJ, Vis AN,
Hoedemaeker RF, et al. Prostate cancer detection at low prostate specific
antigen (2000) J Urol 163:806-812. 23. Gohagan JK, Prorok PC, Hayes RB, Kramer BS and Prostate, Lung,
Colorectal and Ovarian Cancer Screening Trial Project Team. The Prostate, Lung,
Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer
Institute: history, organization, and status (2000) Control Clin Trials
21:251S-272S. 24. Schröder H, Denis LJ, Roobol M and all participants of the ERSPC.
The story of the European randomized study of screening for prostate cancer
(2003) Brit J Urol Internatl 92:1-13. 25. Auvinen A, Rietbergen JBW, Denis LJ. Prospective evaluation plan
for randomized trials of prostate cancer screening (1996) J Med Screening
3:97-104. 26. Andriole GL, Crawford ED, Grubb L, Buys SS, Chia D, et
al. Prostate cancer screening in the randomized Prostate, Lung,
Colorectal and Ovarian Cancer Screening Trial: Mortality results after 13 years
of follow-up (2012) J Natl Cancer Inst 104:125-132. 27. Zhang L, Chang H, Strauss GM. PSA (Prostate-Specific-Antigen)
screening to improve outcome in prostate cancer (PC): Reanalysis of the
Prostate-Lung-Colorectal-Ovary (PLCO) randomized controlled trial (RCT) (2018)
J Clin Oncol 36 (published online before print). 28. Schröder FH., Hugosson J., Roobol MJ. et al Screening
and prostate cancer mortality in a randomised European study. N Engl J Med.
360(13):1320-1328. 2009. 29. Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, et al.
Prostate-cancer mortality at 11 years of follow-up (2012) N Engl J Med.
366:981-990. 30. Schröder FH., Hugosson J., Roobol MJ. et al. ERSPC
Investgators. Screening and prostate cancer mortality: results of at 13 years
of follow-up. Lancet. 384(9959):2027-35. 2014. 31. Schroder FH, Raaijmakers R, Postma R, van der Kwast TH andRoobol
MJ. 4-year prostate specific antigen progression and diagnosis of prostate
cancer in the European Randomized Study of Screening for Prostate Cancer,
section Rotterdam (2005) J Urol 174:489-494. 32. Roobol MJ, Kerkhof M, Schröder FH, Cuzick J, Sasieni P, et al.
Prostate cancer mortality reduction by prostate-specific antigen–based
screening adjusted for nonattendance and contamination in the European
Randomized Study of Screening for Prostate Cancer (ERSPC) (2009) Eur Urol
56:584-591. 33. Crawford ED, Ventii K, Shore ND. New biomarkers in prostate cancer
(2014) Oncology (Williston Park) 28:135-142. 34. Cuzick J, Thorat MA, Andriole G, Brawley OW, Brown PH, et al.
Prevention and early detection of prostate cancer (2014) Lancet Oncol 15:484-492. 35. Ilic D, OConnor D, Green S. Wilt TJ. Screening for prostate
cancer: an updated systematic review (2011) Brit J Urol Int 107:882-891. 36. Popiolek M, Rider JR, Andrén O, et al.: Natural history of early,
localized PCa: a final report. Eur Urol 63:428-435. 2013. 37. Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, et al.
Prostate-Specific Antigen-based screening for prostate cancer: evidence report
and systematic review for the US Preventive Services Task Force (2018) JAMA
319:1914-1931. 38. Ablin RM. The great prostate hoax: How big medicine hijacked the
PSA test and caused a public health disaster (2014) Palgrave Macmillen, United
Kingdom. 39. Welch HG, Black WC. Over diagnosis in cancer (2010) J Natl Cancer
Inst 102:605-613. 40. Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, et
al. Over diagnosis and overtreatment of prostate cancer (2014) Eur Urol
65:1046-1055. 41. Macefield RC, Metcalfe C, Lane JA. et al. Impact of prostate
cancer testing: an evaluation of the emotional consequences of a negative
biopsy result (2010) Br J Cancer 102:1335-1340. 42. Morrison AS. Screening in chronic disease (1985) Oxford University
Press, Oxford, USA. 43. Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, et al. Lead
time and over diagnosis in prostate-specific antigen screening (2009) J Natl
Cancer Inst 101:374-383. 44. Hakama M, Miller AB, Day NE. Screening for the uterine
cervix (1986) IARC Sci publ 76. 45. Moyer VA, U.S. Preventive Services Task Force. Screening for
prostate cancer: U.S. Preventive Services Task Force recommendation statement
(2012) Ann Intern Med 157:120-134. 46. US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK,
Bibbins-Domingo K, et al. Screening for Prostate Cancer: US Preventive Services
Task Force Recommendation Statement (2018) JAMA 319:1901-1913. 47. Basch E, Oliver TK, Vickers A, Thompson I, Kantoff P, et al.
Screening for prostate cancer with prostate-specific antigen testing: American
Society of Clinical Oncology Provisional Clinical Opinion (2012) J Clin Oncol
30:3020-3025. 48. Rendon RA, Mason RJ, Marzouk K, Finelli A, Saad F, et al. Canadian
Urological Association recommendations on prostate cancer screening and early
diagnosis (2017) Can Urol Assoc J 11:298-309. 49. Loeb S. Will changes to prostate cancer screening guidelines
preserve benefits and reduce harm? (2017) Eur Urol 71:66-67. 50. Fornara P, Theil G, Schaefer C. Benefits and risks of prostate
cancer screening (2014) Oncol Res Treat 37:29-37. 51. Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P et al. The
excess burden of side-effects from treatment in men allocated to screening for
PCa (2011) Eur J Cancer 47:545-553. 52. Davidson P, Gabbay J. WHO Regional Office for Europe. Should mass
screening for orostate be introduced at national level? (2004) Health Evidence
Network. 53. Advisory Committee on Cancer Prevention. Recommendations on cancer
screening in the European Union (2000) Eur J Cancer 36:1473-1478. 54. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, et al.
EAU guidelines on prostate cancer. part 1: screening, diagnosis and local
treatment with curative intent - update 2013 (2014) Eur Urol 65:124-137. Prostate Cancer, Opportunistic Screening, Organized
ScreeningScreening for Prostate Cancer: To Screen or Not To Screen? A Review of The State-of-The-Art
Abstract
Full-Text
Introduction
The Burden of Prostate
Cancer
Risk Factors, Natural
History, Pathology
Methods of Prostate
Screening
Digital Rectal Examination (DRE)
Trans Rectal Ultra Sonogray (TRUS)
Prostate-Specific Antigen (PAS)
Benefit-To-Harm Ratio
Discussion
Conclusion
References
Citation: Döbrőssy L. Screening for prostate cancer: to screen or not to screen? a review of the state-of-the-art (2019) Edelweiss Cancer OA
1: 19-24
Keywords