Research Article :
Chidi Okorie Onwuka and Ima-Obong A Ekanem Results:
The overall sensitivity, specificity, positive predictive value and negative
predictive value for VIA were 100%,80%,76.9%, and 100%, respectively compared
to 80%, 100%, 100%, and 88.2% for conventional Pap smear. Visual inspection of
the cervix with acetic acid for cervical cancer screening is not specific but
has a high negative predictive value. Cervical
cancer is the commonest malignant tumour of the female genital tract
and one of the leading causes of cancer mortality in women worldwide with a
global annual incidence of over 500,000 [1,2]. Greater than 85% of these new
cases and about 88% of these cancer related deaths occur in resource-poor countries
[2]. It is the second most common cause of cancer related deaths in regions of
the world where women do not have access to regular gynaecological care and
screening [3]. Visual inspection with
acetic acid requires minimal equipment, does not require any laboratory, can be
performed by non-physicians with adequate training, is inexpensive and yields
immediate results. The WHO has endorsed VIA for cervical cancer screening in
less developed countries with non-existent or poorly executed cervical cancer
prevention programmes since about 80% of all cervical cancer cases occur in
these countries [8]. Visual inspection with acetic acid is not without its draw
backs. Acetowhite lesions are not unique to cervical precancerous lesions. This
generates false positive cases that can lead to overtreatment which can in turn
overwhelm the treatment centres [8]. Also, it is subjective since it depends on
the clinician’s interpretation of what is seen, and is not an appropriate
screening method for postmenopausal women due to fibrosis and leucoplakia of
the cervix [8]. This study was carried
out to compare the sensitivity, specificity, positive predictive value, and
negative predictive value of visual inspection with acetic acid and Pap smear
of the cervix as cervical cancer screening methods among HIV-negative and HIV-positive
women in this region of Nigeria with high HIV prevalence. This comparative
cross-sectional study was carried out in the cytology clinic of University of
Uyo Teaching Hospital, Uyo after approval of the study protocol by the Ethical
committee of the hospital. A total of 461
ever-married or sexually active consenting women were recruited for this study
by word of mouth. Patients bleeding per vaginum, patients with previous
abnormal Pap smear result and pregnant
women were excluded. The results of 449 participants (97.4%), comprising 226 HPW and 223 HNW
were suitable for statistical analysis. Women who did not come back for a
cervical biopsy after an abnormal Pap result or a positive VIA were excluded in
the calculation for sensitivity and specificity. Table 1
shows the socio-demographic characteristics of the study participants who were
aged between 18-60 years with a mean age of 35.24±9.26 years and 35.63±8.44
years in the control- and case- participants respectively. More than half of
the HNW were married (64.1%) and had a secondary or tertiary level of education
(75.6%). Only about one-third of the HPW were married (36.7%) but most of them
also had secondary or tertiary level of education (76.1%). Most of
the women in this study attained menarche at or below
14 years of age (72.6% of the HNW and 67.3% of the HPW) and also had their
sexual debut at or below 18 years (55.2% of the HNW and 68.1% of the HPW) but
most of the HPW (57.1%) had four and above lifetime number of sexual partners
unlike the HNW (39.2%). The study shows that majority of the participants have
a parity between 0 and 2 (57.8% of controls and 69.5% of cases). Only one-tenth
of the study participants in both groups had ever used combined oral
contraceptive pills while about one-third of them
occasionally take alcohol. Most of the study participants are non-smokers
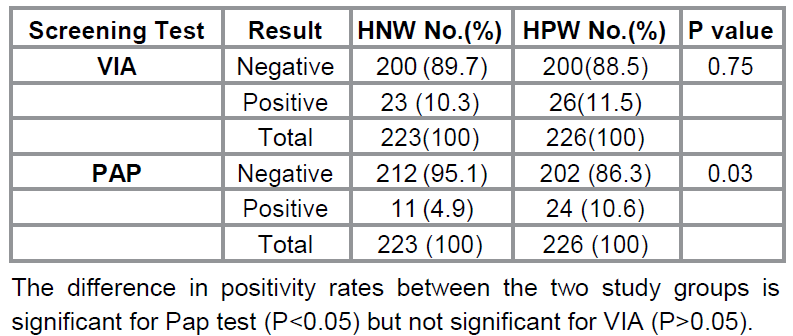
(99.6% of HNW and 99.1% of HPW). Table 2 shows
the positivity rates of VIA and Pap test. Twenty six out of the 226
case-participants (11.5%) and 23 of the 223 control-participants (10.3%) were
positive by VIA, while 10.6% of the case-participants (24 out of 226) and 4.9%
of the control-participants (11 out of 223) were detected positive by Pap test.
A positive Pap test was considered ASCUS or worse lesions. The difference in
positivity rate between the two study groups is significant for Pap test
(P<0.05) but not significant for VIA (P>0.05). Further
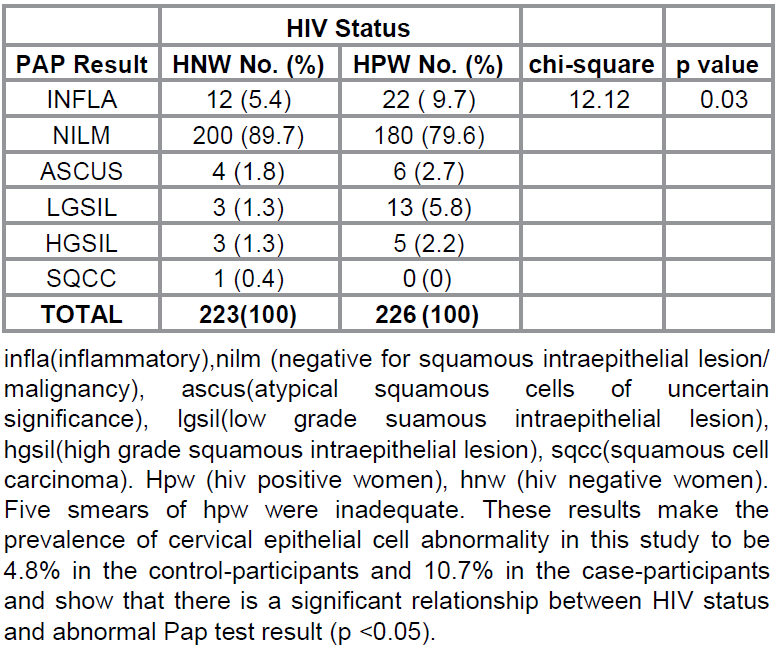
analysis shows the distribution of the Pap test results in the study population
(Table 3). Out of the 231 HPW, 22 (9.7%) had an inflammatory smear and the rest
sub-classified as NILM (180 cases, 79.6%), ASCUS (6 cases, 2.7%), LGSIL
(13cases, 5.6 %), HGSIL (5 cases, 2.2%). Five of the HPW (2.2%) had inadequate
smears due to scant cellularity and excessive mucus obscuring the squamous
epithelial cells. There was no case of cervical cancer in the
case-participants. Twelve of the HNW (5.4%) had an inflammatory smear while the
results of the rest were classified as NILM (200 cases, 89.7%), ASCUS (4 cases,
1.8%), LGSIL (3 cases, 1.3%), HGSIL (3 cases, 1.3%), and Squamous cell
carcinoma (1 case, 0.4%). These
results make the prevalence of cervical epithelial cell abnormality in this
study to be 4.8% in the control-participants and
10.7% in the case-participants and show that there is a significant
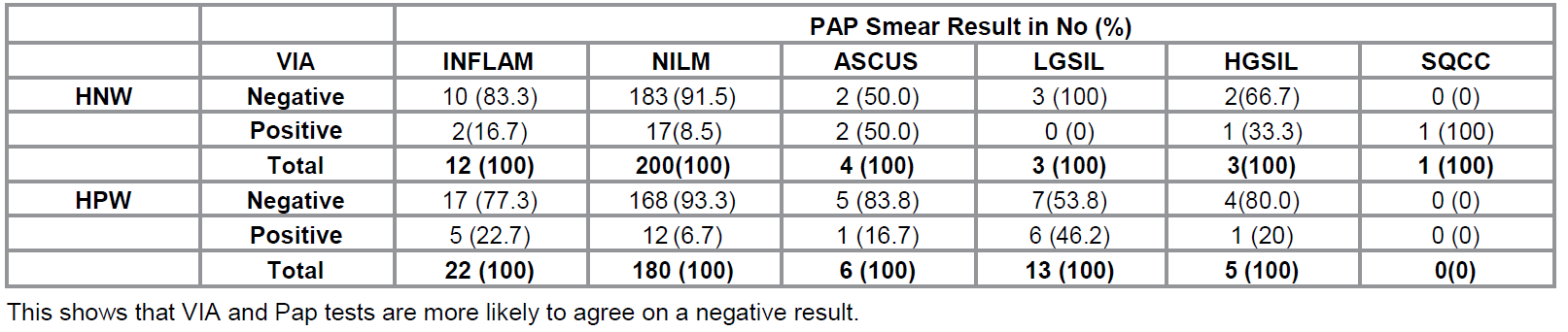
relationship between HIV status and abnormal Pap test result (p <0.05). Table 4 shows the distribution of VIA results in relation to Pap
test results. In the control group, VIA was positive in 16.7% of the
inflammatory smears and 8.5% of the NILM. Fifty per cent of the ASCUS, 100% of
the LGSIL and 66.7% of HGSIL were detected negative by VIA. The SQCC was
positive by VIA. Among the HPW, 22.7% of the inflammatory smear and 6.7% of
NILM were positive on VIA while 83.3% of ASCUS, 53.8% of LGSIL and 80% of the
HGSIL were negative on VIA. This shows that VIA and Pap tests are more likely to
agree on a negative result. All the cases that were negative on both VIA and Pap tests were
confirmed truly negative by biopsy (normal cervices, inflammatory, squamous
papilloma, endocervical polyp). All the cases that were positive on both VIA
and Pap tests were confirmed truly positive on biopsy (CIN-1, CIN-2, CIN-3, and
SCC). Two out of the 5 cases that were VIA positive but Pap negative were
confirmed positive by biopsy (1 CIN-1 and 1 CIN-2) while 3 were reported
negative (2 normal, 1 Nabothian cyst). When the HIV status and only positive screening tests were considered, 7
out of the 9 VIA positive subjects biopsied in the control group (77.8%) and 3
out of the 4 VIA positive patients biopsied in the case-participants (75%) were confirmed positive. All the
positive Pap smears biopsied in the two groups (6 control- and 2
case-participants) were confirmed positive by biopsy. This shows that Pap test
is more specific than VIA in both groups of study participants. Table 1: Socio-demographic and Clinical characteristics. Table 2:
Positivity rate of VIA and
Pap test in the study-participants. Due to the unique association between HIV and HPV in the pathogenesis of cervical cancer, regions with high HIV
prevalence also have high rates of cervical cancer since women comprise about
50% of adults living with HIV/AIDS [9,10].
Many studies have been done to compare the performance of VIA and Pap smear as
cervical cancer screening tools with a wide range of results. To the best of
our knowledge, no published data exist for such a study in this region of
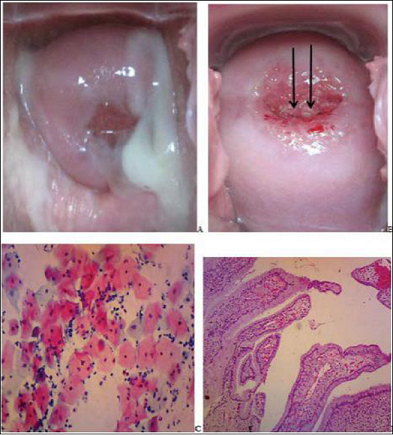
Nigeria with a high prevalence of HIV infection (Figure 1). The
socio-demographic characteristics of the study participants in this study are
similar to those noted in related studies [11-13]. This study showed that VIA was positive in 26 (11.3%) of the HPW and 23
(10.3%) of HNW while Pap smear cytology showed epithelial cell abnormality in
24 (10.7%) of the HPW and 11(4.8%) of the HNW. There was no statistically
significant relationship between HIV status and VIA positivity (p=0.75) Table 3: Distribution of Pap results according to HIV status. Table 4:
Distribution of VIA results
in relation to Pap test results and HIV status but HPW
were 2.5 times more likely to be Pap positive than HNW (p< 0.01). These discrepancies
in the positivity of VIA and Pap test when used on the same group of patients
have also been observed in several studies [14-18]. VIA tends to be more sensitive but less
specific and labels more women positive than Pap test. Reports from South African
and Zambian studies have noted a higher VIA positivity in HPW ranging from
32.6% [14] to 43.7% [15], respectively. A study in Botswana by Ramogola-Masire
et al. among 2,175 HPW showed 15.2% VIA positivity that were histologically
confirmed CIN [16]. Similar VIA studies
in Mali and Tanzania conducted on women regardless of their HIV status have
also reported varying positivity ranging from 2.6% [17] to 7.1% [18] respectively. The discrepancies noted with these VIA
studies may be attributable to a lack of reproducibility of this screening
method, differences in exclusion criteria and age differences. Also regional
variation in HIV prevalence, HPV and cervical neoplasia burden may account for the regional discrepancies observed in VIA
studies. The rate of VIA positivity
observed in this present study is however supported by studies that recorded
similar frequencies
[19,20]. The low positivity of VIA noted in this
study might be because of the low prevalence of abnormal cervical cytology in
both the cases and control groups used in this study, recording only distinct
acetowhite areas as positive and not including cervix with postmenopausal leukoplakia nor faint and suspicious whitish appearance. The problem of
inter-observer variability is also relevant [21]. Using
biopsy positive cases as gold standard, this study showed that VIA is more
sensitive but less specific than Pap test. A threshold of CIN-1 or worse lesion was considered a positive biopsy. The
sensitivity of VIA in the total study population regardless of HIV status was 100% while that for Pap test
was 80%. Combining VIA and Pap test gave a sensitivity of 100%. When the HIV
status was considered, the sensitivity of VIA and VIA+PAP was 100% in both
groups of women while that for Pap test was 85.7% in the HNW and 66.7% in the
HPW. This higher sensitivity of VIA noted in this study is supported by
findings in several studies that have yielded a wide range of results. The values
for the sensitivity of VIA reported in literature range from 60 to over 90%
while that for cytology span from 23% to 99% [22,23]. Rana et al reported sensitivities of 31.6% vs
78.2% for VIA and Pap smear respectively while Gravitt et al found 59.7% vs 57.4% in a similar study
[24,25]. The sensitivity pattern seen in
this study is similar to that observed by Akinola et al in Lagos, Nigeria [12]. Various reasons account for the
varied result in most studies comparing the sensitivity of VIA and Pap smear in
biopsy positive cases. One such factor is the threshold of positive
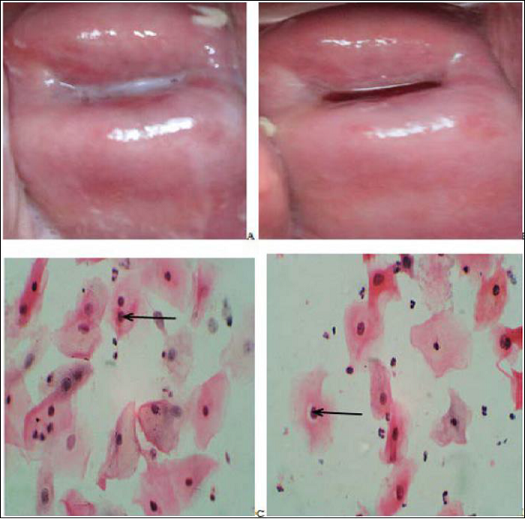
histopathology (Figure 2). This study used a threshold of CIN 1 or worse while other studies used CIN 2 or worse lesions.
Also, the problems with conventional Pap smear may contribute to the lower
sensitivity of Pap test observed in this and other studies. Conventional Pap
test has a lesser sensitivity in detecting precancerous lesions of the cervix
due to potential limitations which include inadequate transfer of cells to
slide, in homogeneous distribution of abnormal cells and obscuring factors such
as inflammatory
cells and blood [26]. Because
of this limitation, a potentially dysplastic
lesion can be wrongfully considered an inflammatory smear
thereby causing a lower sensitivity in comparison studies. This shows the
advantage of liquid-based cytology in Pap test. Another important factor is
inter-observer differences in reporting a positive VIA. This study reported
only cervixes or lesions on the cervix that show distinct whitening after one
minute of acetic acid application as positive. This may account for the
positivity rate of VIA obtained in this study. This buttresses the importance
of adequate training and experience in performing a VIA. Training protocol
needs to be developed in remote areas for adequate training and evaluations to
maintain the expertise of health workers involved in administering VIA. The specificities of VIA, Pap test, and VIA+Pap test in the total study
population were 80%, 100% and 100%, respectively. According to HIV status,
these values were 81.8%, 100%, and 100% in the HNW and 75%, 100, and 100% in
the HPW respectively. These findings show that Pap test is more specific than
VIA and are supported by reports from other Nigerian [11-13] and
international studies [25-27]. The commonly reported specificity for VIA ranges
from 60 to over 90% while that for conventional Pap smear spans from 7% to 97%
for specificity [22,23]. The reasons for the false positive cases noted in this
study included postmenopausal state, postmenopausal
cervicitis, chronic cervicitis, nabothian cysts, and squamous
papilloma. These reasons have been noted by the IARC and other studies
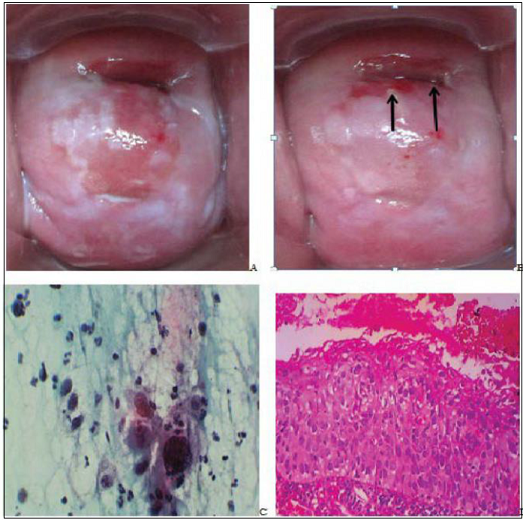
[28,29] (Figure 3). In this
present study, it was observed that combining VIA and Pap smear increased the
sensitivity of Pap test and the specificity of VIA. This is similar to the
finding by Mahmud et al in Pakistan in a study done to compare VIA and Pap
smear in cervical cancer screening [30] (Figure 4). This finding is important
and points to the fact that VIA can be utilised in two ways in resource-limited
and developing countries. First, due to its higher
sensitivity, it can be utilized in mass screening exercise to triage women who
would benefit from further evaluation by other more specific methods of
cervical cancer screening. Secondly, due to the improved sensitivity and
specificity of the combined tests, VIA can be used to complement conventional
Pap smear in opportunistic screening of women with no assurance of follow up
contact and in tertiary institutions with limited cytopathologist and absent
liquid-based Pap smear preparations. One major problem with VIA is its lack of specificity. VIA is more
sensitive but less specific than Pap smear in detecting precancerous lesions of
the cervix and if used as an alternative to Pap smear may result in increased
referral for colposcopy and biopsy which could be burdensome in some developing countries.
Also, in settings where the ‘see-and-treat approach is adopted without
confirmation using other screening modalities, VIA would lead to over
treatment. However, in resource-poor settings, due to the increased risk of HPV
infection in HIV- infected women, and the accelerated progression from cervical
neoplasia to invasive cancer in HPW, the benefits of overtreatment, increased
referral for colposcopy and biopsy far outweigh the danger of failure to
diagnose a potentially dysplastic lesion. More so, to prevent an overtreatment,
VIA can be used in a large scale programme to triage women who would benefit
from further evaluation including VILI (visual inspection with Lugol’s Iodine),
VIAM (visual inspection with acetic acid under magnification), Pap smear, colonoscopy
and directed biopsy, and HPV DNA testing. This
study also showed that VIA has a higher negative predictive value (NPV) than
Pap test. The NPV of VIA, Pap test, VIA+Pap in the total study population in
this study were 100%, 88.2%, and 100%, respectively. These values were 100%,
91.7%, and 100 in the control group, and 100%, 80%, and 100% in the
case-participants, respectively. Other studies have reported a NPV for VIA to
be close to or more than 90% which support the finding in this study [22-25]. Also,
the negative percentage agreement between VIA and Pap test was higher than the
positive percentage agreement in this study (93.1% vs 65.4%). This further
supports that VIA has a high NPV. This high NPV of VIA is of benefit in
resource-limited areas as a woman who tests negative by VIA can be reassured
and sent home without further evaluation with more costly protocol. This is
important because the vulnerable groups for cervical cancer are the poor, rural
dwellers, and HPW [31]. The
positive predictive value (PPV) for VIA, Pap test, and VIA+Pap in the total
study population in this study were 76.9%, 100%, and 100%, respectively.
Considering the HIV status, these values are 77.8%, 100%, and 100% in the HNW
and 75%, 100%, and 100% in the HPW, respectively. The reported value for PPV of
VIA in previous studies ranges from 3.8% to 90% [32]. This finding of
comparable PPV of VIA and Pap test in this study differs from that seen in most
of the work cited. This might be because in this
study, only distinct acetowhite lesions where reported as positive thereby
limiting sources of false positive cases seen in most studies. Conditions that
have been reported to cause a false positive VIA include cervical
polyp, inflammation, postmenopausal leukoplakia, and metaplasia [33, 34]. In this
study, 5 out of the 22 inflammatory smears in HPW and 2 out of 10 inflammatory
smears in the HNW were reported positive by VIA but none of the positive VIA
was diagnosed inflammatory by biopsy. In comparing VIA and biopsy, no false
positive case was due to cervicitis in this study. The results of this present study and
that of other reported studies show that VIA is comparable to cytology as a cervical
cancer screening tool and can be used in resource poor settings in both HPW and
HNW for cervical screening. Because of its affordability, simplicity
and rapidity of performance, VIA has emerged as one of the promising
alternatives to Pap smear in low-resource settings for cervical cancer
screening. VIA is cheap, easy to perform by a trained nurse and the result is
available immediately allowing the triaging of women who may require further
investigation to identify and treat cervical lesions. Triaging would help
reduce the workload on the limited number of cytotechnologists and
cytopathologists in resource-poor settings if a large scale cervical cancer screening
program is to be carried out. Furthermore, in places where cytology services
are available, VIA can be used as an adjunct to improve on the sensitivity of
conventional cervical cytology in detecting cervical neoplasia since
conventional Pap smear is noted to be associated with a high rate of false
negativity from sampling and interpretative errors [35]. The major concern about VIA is its low
specificity [high rate of false positivity] which may lead to over referral and
overtreatment but the danger of missing a potentially dysplastic lesion far
outweighs the cost of an overtreatment. Moreover overtreatment can be prevented
by confirming positive cases by other methods before commencement of treatment.
This
study shows that cervicitis has no significant effect on the result of
VIA. Only 22.7% of the inflammatory
smears in the HPW and 16.7% of the inflammatory smears in the HNW were detected
positive by VIA. Using biopsy as gold standard, none of the positive VIA was
detected as inflammatory. This finding is supported by studies that have shown
no association between a false positive VIA to specific genital tract
infections other than HPV [34]. Factors
noted to be associated with VIA positivity in this study includes age and Pap
smear positivity. It was also noted that postmenopausal women are more likely
to present with a false positive VIA. In the age distribution of VIA result,
older women are more likely to present with a positive VIA (p<0.05). This
finding is similar to that seen in IARC studies [28]. The IARC recommends that
VIA should not be used in postmenopausal women for cervical cancer screening
[28]. VIA is
more sensitive but less specific than conventional Pap test. This finding is
similar to that reported in the literature. VIA could be used as an alternative
to cytology in resource poor settings in both HPW and HNW for primary cervical
screening. Due to its sensitivity, affordability, simplicity, and rapidity of
performance, VIA can be used for mass cervical
cancer screening. This would help triage women for further evaluation before
applying the appropriate treatment. This study therefore does not support the
‘see-and treat’ approach in cervical cancer management because VIA is not
specific. Also, the ‘see-and-treat’ approach will prevent generation of data to
know what is being treated and the depth of the lesion that is being treated
may not be reached, giving a false sense of safety. The
authors are grateful to the management of University of Uyo Teaching Hospital
for approving this study and to the personnel of the HIV clinic who contributed
to the success of this study. We are grateful to Mr Jezreel Ajayi for technical
assistance and for performing statistical analysis. 1.
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. Estimates of
Worldwide burden of cancer in 2008, GLOBOCAN 2008 (2011) Int J Cancer 127: 2893-2917. 2.
Ferlay J, Soerjomataran I, Ervikm M, Dikshit R, Eser R, et al. GLOBOCAN
2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No.11 (2013)
International Agency for Research on Cancer, Lyon, France. 3.
Ntekim A. Cervical cancer in sub-Sahara Africa. (2012) In: Topics on
cervical cancer with an advocacy for prevention, Rajamanickan R (edtr) In-Tech
Pp: 51-74. 4.
Abd El All HS, Refaat A, Dandash K. Prevalence of Cervical neoplastic
lesions in Egypt: National Cervical Cancer Screening Project (2007) Infectious
agents and cancer 2:12. 5.
Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, et al. Human
papillomavirus prevalence and type distribution in invasive cervical cancer in
Sub-Saharan Africa (2014) Int J Cancer 134:1389-1398. 6.
Del Mistro A, Salamanca HF, Trevisan H, Bertorelle R, Parenti A, et al.
Human papillomavirus typing of invasive cervical cancers in Italy (2006)
Infectious Agents and Cancer 1: 9. 7.
Castellsague X, Munoz N. Cofactors in HPV carcinogenesis of parity, oral
contraceptive and tobacco smoking (2003) J Natl Cancer Inst Monogra Pp: 20-28. 8.
Alliance for cervical cancer prevention (2004) Planning and implementing
cervical cancer prevention and control programs. A manual for managers. Seattle,
ACCP. 9.
Anderson J, Lu E, Sanghvi H, Kibwana S, Lu A. Cervical Cancer Screening
and Prevention for HIV-infected women in developing world (2014) In: Cancer
prevention from mechanism to translational benefits, Georgakilas AG (edtr). 10.
National Centre for HIV/AIDS (2009) Dermatology and STD annual report. 11.
Albert SO, Oguntayo OA, Samaila MOA. Comparative study of visual
inspection of the cervix using acetic acid (VIA) and Papanicolaou (Pap) smears
for cervical cancer screening (2012) ecancer 6:262. 12.
Akinola OI, Fabamwo AO, Oshodi YA, Banjo AA, Odusanya O, et al. Efficacy
of visual inspection of the cervix using acetic acid in cervical cancer
screening: a comparison with cervical cytology (2007) J Obstet Gynaecol 27: 703-705. 13.
Akinwuntan AL, Adesina OA, Okolo CA, Oluwasola OA, Oladokun A, et al.
Correlation of cervical cytology and visual inspection with acetic acid in
HIV-positive women (2008) J Obstet Gynaecol 28: 638-641. 14.
Kuhn L, Wang C, Tsai WY, Wright TC, Denny L. Efficacy of human
papillomavirus-based screen-and-treat for cervical cancer prevention among
HIV-infected women (2010) AIDS 24: 2553-2561. 15.
Pfaender KS, Mwanahamuntu MH, Sahasrabuddhe W, Mudenda V, Stringer JS, et
al. Management of cryotherapy-ineligible women in a “screen-and-treat” cervical
cancer prevention program targeting HIV-infected women in Zambia: Lessons from
the field (2008) Gynecol Oncol, 110: 402-407. 16.
Ramogola-Masire D, de Klerk R, Monare B, Ratshaa B, Friedman HM, et al.
Cervical cancer prevention in HIV-infected women using the “see-and-treat”
approach in Botswana (2012) J Acquir Immune Defic Syndr, 59: 308-313. 17.
Teguete I, Muwonge R, Traore CB, Dolo A, Bayo S. Can visual cervical
screening be sustained in routine health services? Experience from Mali, Africa
(2012) BJOG 119: 220-226. 18.
Ngoma T, Muwonge R, Mwaiselage J, Kawegere J, Bukori P, et al. Evaluation of
cervical visual inspection screening in Dar es Salaam, Tanzania (2010) Int J
Gynaecol Obstet 109: 100-104. 19.
Hedge D, Shetty H, Shetty PK, Rai S, Manjeera L, et al. Diagnostic value
of VIA comparing with conventional Pap smear in the Detection of colposcopic biopsy
proved CIN (2011) NJOG Pp: 7-12. 20.
Horo A, Jaquet A, Ekouevi DK, Troure B, Coffie B, et al. Cervical cancer
screening in Cote d’Ivoire: operational and clinical aspects according to HIV
status (2012) BMC Public Health 12:237. 21.
Sherigar B, Dalal A, Durdi G, Pujar Y, Dhumale H. Cervical cancer
screening by VIA- Interobserver variability between Nurse and Physician (2010)
Asian Pacific J Cancer Prev 11: 619-622. 22.
Rodriguez-Reyes ER, Cerda-Flores RM, Quinonez-Perez JM,
Velasco-Rodriguez V, Cortes-Gutierrez EI. Acetic acid test: A promising
screening test for early detection of cervical cancer (2002) Anal Quant Cytol
Histol 24: 134-136. 23.
Sankaranarayanan R, Rajkumar R, Theresa R, Esmy PO, Mahe C, et al:
Initial results from a randomized trial of cervical visual screening in rural
south India (2004) Int J Cancer 109: 461-467. 24.
Rana T, Zia A, Sher S, Tariq S, Asghar F. Comparative evaluation of Pap
smear and Visual inspection of acetic acid in acid (VIA) in Cervical Cancer
Screening Program in Lady Willingdon
Hospital , Lahore (2010) Annals of King Edward Medical University 16: 104-107. 25.
Gravitt PE, Paul P, Katki HA, Vendantham H, Ramakrishna G, et al.
Effectiveness of VIA, Pap, and HPV DNA testing in a cervical cancer screening
program in a peri-urban community in Andhra Pradesh, India (2010) PLoS One
5:e13711. 26.
Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, et al. Pooled
analysis of the accuracy of Five cervical cancer screening tests assessed in
eleven studies in Africa and India (2008) Int J Cancer 123: 153-160. 27.
Duraisamy K, Jaganathan KS, Bose JC. Methods of detecting cervical
cancer (2011) Advances in Biological Research 5: 226-232. 28.
IARC Screening Group. Anatomical and Pathophysiological basis of Visual
Inspection with acetic acid (VIA) and with Lugol’s Iodine (VILI). 29.
Goel A, Gandhi G, Batra S, Bhambhari S, Zutshi V,. Visual inspection of
the cervix with acetic acid for cervical intraepithelial lesions (2005) Int J
Gynaecol Obstet 88: 25-30. 30.
Mahmud G, Tasmin N, Iqbal S. Comparison of Visual Inspection with acetic
acid and Pap smear in cervical cancer screening at a tertiary care hospital
(2013) JPMA 63: 10-13. 31.
WHO (2010) Cervical cancer summary reports. 32.
Hasanzadeh M, Esmaeih H, Tabaee S, Samadi F. Evaluation of visual
inspection with acetic acid as a feasible screening test for cervical neoplasia
(2011) J Obstet Gynaecol Res 37:1802-1806. 33.
Cagle AJ, Hu SY, Sellors JW. Use of an expanded gold standard to
estimate the accuracy of colposcopy and visual inspection with acetic acid
(2010) Int J Cancer 126: 156-161. 34.
Davis-Dao CA, Cremer M, Felix J, Cortessis VK. Effect of cervicitis on visual inspection
with acetic acid (2008) J Low Genit Tract Dis 12: 282-286. 35.
Koss LG. Cervical (Pap) smear (1993) Cancer 71: 1406-1412. *Corresponding author:
Chidi Okorie Onwuka,
Department of Pathology, University of Uyo
Teaching Hospital, Uyo, Akwa Ibom State,
Nigeria, Tel: +234 (0) 8035473424,
E-mail: dronwuka@yahoo.com
Cervical cancer, Pap smear, VIA, CytologyThe Utility of Visual Inspection with Acetic Acid in Cervical Cancer Screening
Abstract
Methodology:
Between March, 2013 and March, 2014, 461 consenting women, comprising 231 HIV
positive women (HPW) and 230 HIV negative women (HNW) were recruited and
screened for cervical cancer using conventional Pap smear and VIA
simultaneously in University of Uyo Teaching Hospital. The Pap smear findings
were classified using the 2001 Bethesda system. Patients with a positive Pap
smear or abnormal VIA findings were recalled for biopsy. The results of the two
tests were compared using biopsy as the gold standard.
Conclusion: This study does not
support a “see-and-treat” approach in cervical cancer management using VIA only.
In resource-challenged areas, VIA can be applied on a large scale basis in
primary screening for cervical cancer so as to triage, women who will benefit
from further evaluation before applying the appropriate treatment. Full-Text
Introduction
Most cases of cervical
cancer are Human
papilloma virus (HPV) associated [4-6]. Early onset of
sexual activity, early age at first pregnancy, high parity and multiple sexual
partners are associated with the risk of HPV infection [7]. Other risk factors
include presence of other sexually transmitted diseases, low socioeconomic
class, cigarette smoking and immunosuppression from any cause, vitamin
deficiency, and long term oral contraceptive use [7].
Unlike most cancers,
invasive cervical cancer is potentially preventable because it is preceded by
long precancerous stages which are most often detected by cervical smear. In
developed countries, routine and organized cytology-based screening programmes
with adequate treatment of precancerous lesions have dramatically reduced the
incidence and mortality of cervical cancer. These require a reliable health
care infrastructure, political support, adequate number of trained personnel,
and multiple clinic visits. All of these factors make the implementation of
such programmes difficult in low-resource countries where other health needs
are competing for the available resources. This makes the development of
alternative methods for cervical cancer screening necessary in such
resource-poor settings. Visual inspection of the cervix with acetic acid (VIA)
is one of such alternatives and requires the application of 3% to 5% acetic
acid to the cervix which is then examined after one minute for the
characteristic acetowhite lesions suggestive of cervical neoplasia.Materials and Methods
The study
participants were educated about cervical cancer, the screening methods,
objective of the study, and the possibility of an abnormal result. Those who
were willing to take part in the study signed a written informed consent.
A questionnaire
was administered to obtain information on each participant’s socio-demographic
factors and relevant risk factors. Participants’ confidentiality was ensured.
The procedure was explained to each of the participants. The Pap smear was
taken while observing standard protocol and promptly immersed in 95% ethyl
alcohol fixative contained in a coplin jar. With the vaginal speculum still in
place after collecting the sample for Pap smear, a cotton swab soaked in 5%
freshly prepared acetic acid was applied to the cervix. The cervix was examined
after one minute for the characteristic aceto-white appearance typical of
cervical neoplasia by unaided naked eye examination. Photographs of the cervix
were taken before and one minute after applying the acetic acid using a Canon
Power shot A80 5X optical zoom 16.0 mega pixels camera. Findings on VIA were
recorded immediately and classified based on the criteria from International
Agency for Research in Cancer into Negative or Positive. Lesions that were
positive or suspicious on VIA were biopsied in women who gave consent for the
procedure.
The
alcohol-fixed smeared slides were then stained with Pap stain using standard protocol
at the histopathology laboratory of the hospital. The 2001 Bethesda System
(TBS) of reporting cervical and vaginal cytology was used as the basis for
cytology classification.
Women who had an
abnormal cytology with a normal VIA finding were recalled for biopsy.
The biopsy materials were processed by the formalin fixed paraffin embedded
method using standard protocols. The cervical biopsies were classified according
to the WHO classification of cervical tumors.
The results of the two screening tests for each patient were compared
using the biopsy result as the reference standard. For this, all Pap smears
with the diagnosis of ASCUS or worse lesion were considered positive while all
abnormal VIA findings were considered positive. The threshold of positive
biopsy is CIN-1 and worse lesions
The
data collated were analyzed using statistical package for social sciences
version 20 (SPSS 20) and the results presented as tables. The level of
significance was set at P less or equal to 0.05. The findings of this study
were compared with those of previous studies.Results

Discussion
Conclusion
Acknowledgement
References
Citation: Onwuka CO, Ekanem IA (2017) The
Utility of Visual Inspection with Acetic Acid in
Cervical Cancer Screening. ECOA 103: 7-14 Keywords