Introduction
Dental pulp tissue is capable of innate and adaptive immune responses caused by various mmunological conditions [1-3]. One host-defense system, involving the innate immune response upon exposure to the external environment, is the production of defensins [4]. Human beta-defensins (hBD) are small cationic antimicrobial peptides produced by epithelial cells and expressed by all human mucosa [5] including oral mucosa [6], odontoblasts [7] and pulp cells [8].
The mechanisms of the host immune defense against infections in human dental pulp (HDP) cells are not completely understood and the role that hBD play in protection of these cells has yet to be thoroughly explored.
Human beta-defensins have demonstrated immunologic response against grampositive and -negative bacteria, mycobacteria, fungi, and certain enveloped viruses at low micromolar concentrations [9,10]. Human beta-defensins have antiretroviral activity by inhibiting HIV-1 infectivity of immunocompetent cells [11]. Additionally, hBDs can enhance adaptive immunity by acting as adjuvants and chemoattracting T cells, immature dendritic cells [5], neutrophils [12] and macrophages [13]. Human beta-defensins-2 is mediated through nuclear factor kappa-light-chain-enhancer of activated B cells (NF-KB) and mitogen-activated-protein-kinases (MAPK) pathways [14,15], while hBD-3 is dependent upon epidermal growth factor receptor (EGFR) activation [16,17].
There are many studies in the medical literature that have linked hBD-2 and hBD-3 with cytokine and chemokine production [18-21].
Human beta-defensins-2 are highly expressed when the human dental pulp cells are stimulated with IL-1β or TNFα [22]. Dommisch et al. 2007 [23] reported that hBD-2 stimulation of odontoblasts and dental pulp stem cells led to up-regulation of the IL-6 and IL-8 mRNA.
Most of the above mentioned studies were in-vitro investigations. To date only one clinical dental study investigated the association between inflamed pulp and hBD’s in HDPs [8]. Since bacteria from carious lesions elicit inflammatory and immunological responses in the dental pulp [24], the current authors reasoned that the relative concentrations of hBDs and inflammatory cytokines might modulate the outcome of pulp pathosis. A better understanding of pulpal immune response at different stages of inflammation may allow development of an immune system-based pulp therapy in the future. To begin testing this hypothesis, the current authors examined hBDs and cytokine profiles of symptomatic and asymptomatic irreversible pulpitis in human teeth.
To the best of our knowledge, no previously published work has examined hBDs or cytokine and chemokine profiles involved in endodontic pulpal pathosis. Thus, the aim of this study was to investigate the levels of hBD-2 and hBD-3, and chemokine and cytokine expression levels in pulps from teeth endodontically diagnosed with symptomatic irreversible pulpitis, asymptomatic irreversible pulpitis or normal pulps. We hypothesized that there would be a correlation between hBD’s and the immunoregulatory response in the pulp.
Materials and Methods
Patient selection
This study was approved by the Institutional Review Board (IRB), Case Western Reserve University, Cleveland, Ohio, and written informed consent was granted from all patients. Patients undergoing non-surgical root canal treatment from August 2013 to April 2014 were selected. The investigation did not alter the treatment plan of any patient. Patients were provided with information about the purpose of the study and written informed consent was obtained. Participants had to have met the following criteria: American Society of Anesthesiologists (ASA) physical status 1 or 2, no history of known allergies, not pregnant, nonsmokers, age between eighteen and sixty five, healthy periodontal status and restorable teeth. Exclusion criteria: younger than eighteen years or older than sixty five, patient on antibiotics, patients with any known allergies, pregnancy, diabetes, immunocompromised patients, any periodontal probing depth greater than 5mm or teeth with a furcation or trifurcation involvement, teeth diagnosed with a necrotic pulp, previously initiated endodontic treatment and/or previously endodontically treated teeth.
Each patient’s pulpal and periradicular status were evaluated by cold test (HYGIENIC®; ENDO-ICE®; Coltène/Whaledent Inc., Cuyahoga Falls, OH, USA) and electric pulp test (EPT) (Vitality Scanner; SybronEndo, Orange, CA, USA) to assess pulp vitality. Percussion, palpation, and periodontal examinations were performed. Digital periapical and bitewing radiographs of the tooth in question (Planmeca® ProSensor™; PLANMECA USA, Inc., Roselle, IL, USA) were obtained.
The following type patients were subsequently included: patients diagnosed with symptomatic irreversible pulpitis (SIP) or asymptomatic irreversible (ASIP) pulpitis where excavation of caries resulted in pulpal exposure. The definitions of the SIP and ASIP were described in a previous study [25]. No traumatized teeth were included in this research. As a negative control, samples were also taken from six teeth which had no clinical or radiographic evidence of pulpal and periapical pathosis but needed routine endodontic treatment for prosthodontic reasons. Briefly, the clinical characteristics of the cases included subjective and objective findings which are described
SIP: included sharp pain upon thermal stimulus,
lingeringpain (often 30 seconds or longer after stimulus removal).
ASIP: these cases had no clinical
symptoms and usually respond normally to thermal testing deep caries resulted
in exposure following removal [26].
Control: normal pulp, where the teeth were symptom-free, healthy and free of
caries.
Operative procedure & site selection
The sampling procedure is a modified procedure as described by Martinho et al. 2008, 2015 [27,28]. In brief, teeth were mechanically cleaned and disinfected by 0.12% chlorhexidine (Peridex™; 3M™, USA). After local anesthesia and rubber dam placement, an access opening was made using a sterile size #2 round carbide bur (Dentsply Maillefer, Tulsa, OK, USA) in a high speed hand piece to expose the pulp. An ENDO-Z bur (Dentsply Maillefer, Tulsa, OK) was used to deroof the pulp chamber. Paper points size 35/0.02 taper (Lexicon ®; DENTSPLY Tulsa Dental Specialties, John City, TN, USA) were introduced into the pulp chamber and left for 60 seconds. The procedure was repeated with 4 paper points. The paper points were placed into Eppendorf tube (Eppendorf Tubes®, Lakewood, OH, USA) containing 400 µL of phosphate-buffered saline (PBS) (Gibco® PBS pH7.4,Grand Island, NY, USA) centrifuged at 10000 g at 4°C for 15 minutes and stored at -70°C until use.
Bicinchoninic acid assay (BCA assay)
Bicinchoninic acid assay was first introduced by Smith et al. [29] and is a sensitive methodology for protein quantification [30].
Total proteins in the samples were measured using the BCA protein assay kit (Pierce, Rockford, IL, USA) following the manufacturer’s instructions.
Enzyme-link immunosorbent assay (ELISA)
Levels of hBD-2 and hBD-3 were measured by sandwich enzyme-linked immunosorbent assay (ELISA). Ninety-six-well immunoplates (R&D, Minneapolis, MN) were coated with 100 μL goat anti–hBD-2 or rabbit anti–hBD-3 antibodies (Peprotech, NJ) diluted to 1 mg/L in 0.05 mol/L carbonate buffer, pH 9.6, 4°C, for 18 h. Subsequently, the sample were blocked with 200 μL 1% bovine serum albumin in PBS at room temperature about 20-25°C] for 10 minutes. After washing three times with 200 μL phosphate buffered saline (PBS), 0.01% Tween 20, 50 μL of test samples + 50 μL of PBS per well were added and incubated at room temperature for 60 min. Plates were washed three times with PBS, 0.01% Tween 20 and wells incubated at room temperature with 100 μL biotinylated goat anti-human BD-2 or biotinylated rabbit anti-human BD-3 (Peprotech, NJ) diluted to 0.2 mg/L in PBS, 0.01% Tween 20 for 30 min. Plates were washed three times with PBS, 0.01% Tween 20 and 100 μL/well streptavidin HRP (R&D, Minneapolis, MN) was added. Plates were then incubated at room temperature for an additional 30 min, washed three times as described above, and incubated with 100 μL of Reagent (A+B) (R&D, Minneapolis) in the dark at room temperature for about 15 min. Reactions were stopped by adding 50µL of stop solution (R&D, Minneapolis). Absorbance was measured at 450nm in a microplate reader (Bio-Rad Model 680). Human beta defensins were quantified by simultaneous ELISA using recombinant hBDs as calibrators.
Cytokine and Chemokine measurement by Luminex
The Clinical Translational Science Collaborative Bioanalyte Core utilizes software that allows for the standardization between samples and between studies longitudinally; all values are evaluated using a standard curve which has been validated as a comparison of multiple assays to assure consistency between analyses (found at: http://casemed.case.edu/ctsc/cores/bioanalyte.cfm). The standard curve for sample cytokine and chemokine concentration determination was used on the basis of the standard curve using Bio-Plex Manager 6.1 (Bio-Rad Laboratories, Hercules, CA).
Luminex analysis (Luminex®100 platform; Austin, TX, USA) of the samples was performed using the Cytokine and Chemokine Human 10-plex panel multiplex assay (Novex®, Life technologies™, Grand Island, NY, USA). These included: tumor necrosis factor- α (TNFα), interleukins (IL-1β, IL-6, IL-8, IL-10, IL-17, IL-17F), monocyte chemotactic protein-1 (MCP-1; also known as chemokine (C-C motif) ligand 2 CCL2), macrophage inflammatory protein (MIP-1a, also known as CCL3) and regulated on activation normal T cell expressed and secreted (RANTES, also known as CCL5). Levels of the cytokines were normalized with total protein as measured by BCA assay and expressed as pg/mg of total proteins.
Normalization Methods
Total protein
To normalize the data the levels of the hBDs, cytokines and chemokines were expressed as per mg of total proteins The total concentration of protein was measured in each specimen using a bicinchoninic acid (BCA) assay as per manufacturer’s protocol (Pierce, Rockford IL). This assay has a reported dynamic range of 20–20,000 μg/ml and has a 14.7% mean coefficient of variance for repeat testing across 14 different human and non-human purified protein targets. Specimens were diluted 1:10 and 1:100 in PBS and run in duplicate. Colorimetric detection of test specimens was normalized to background specimens that contain extraction buffer only. Total protein concentration was estimated using an 8-point standard curve and is expressed as μg/ml. The ratio of immune marker concentration to total protein concentration was then calculated and expressed as [pg of immune marker]/[mg of total protein].
Sample calculation
sample size calculation was performed before the beginning of the study using the SAS Power and Sample Size 3.1 of SAS (Statistical Analysis System) software for Windows version 9.1.3 (SAS Institute Inc. Cary, NC, USA). Expecting the minimum correlation of 0.60 with power of 0.80 and alpha of 0.05 and one sided test, the minimum sample size for the experimental cases was 15 root canals.
Statistical analysis
Demographic characteristics were expressed as the means and standard deviations and Chi-square test. Results of hBDs, chemokines and cytokines were normalized by the equation: (cytokine or chemokine or HBD expressed) / total protein (BCA assay). Data were statistically analyzed using Kruskal-Wallis, Wilcoxon Mann-Whitney test and Spearman correlation test. The level of statistical significance was set at 95% confidence interval (p < 0.05), and the statistical analysis was calculated using Prism 6.0 software (GraphPad Prism version 6.0 for Windows, San Diego, CA, USA).
Results
Table 1 details the demographic distribution and the mean age. To determine whether the variables were statistically independent we performed Pearson Chi Square test and the result found not to be significant (χ2 =1.534, p=0.464).

Table 1: Demographic characteristics and endodontic diagnosis of the healthy patients.
Table 2 details the mean, median and standard deviation of levels of hBD’s, cytokines and chemokines in normal, SIP and ASIP groups.
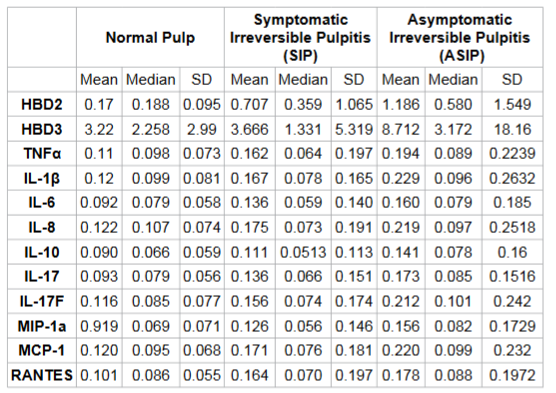
Table 3 details the analysis between levels of normalized hBD-2 in comparison to chemokines, cytokines with statistical analysis. To normalize the data the levels of the hBDs, cytokines and chemokines were expressed as per mg of total proteins. There was no correlation between the levels of hBD-2 in comparison to the cytokines and chemokines in the normal and SIP groups; however, in the ASIP group there was a correlation between the levels of hBD-2 and TNFα, IL-6, IL-8, IL-10, MCP-1, IL-1β, MIP1a, RANTES, IL-17 and IL-17F.
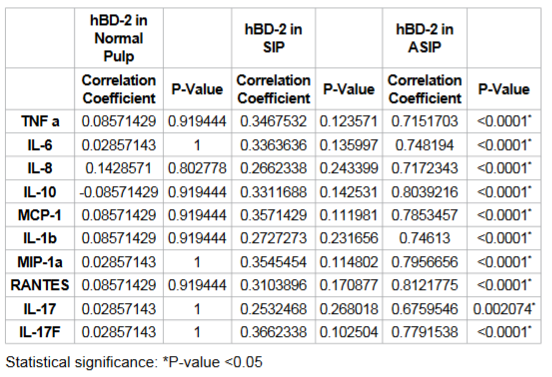
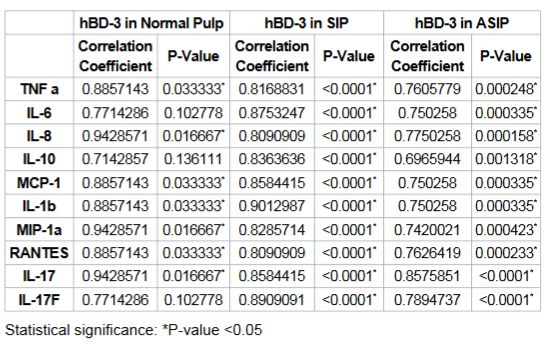
Table 4 details the levels of normalized hBD-3 in comparison to chemokines, cytokines with statistical analysis. In the SIP and ASIP groups there was a correlation with TNFα, IL-6, IL-8, IL10, MCP-1, IL-1β, MIP-1a, RANTES, IL-17 and IL-17F (all the cytokines, chemokines studied). For all groups, there was a correlation with the levels of hBD-3 to TNFα, IL-8, MCP-1, IL-1β, MIP-1a, RANTES and IL-17.
Discussion
To the best of our knowledge, this is the first endodontic clinical study that investigated the role of hBDs in relation to pulpal cytokines and chemokines. Since there have been many medical studies which have linked hBD-2 and hBD-3 with cytokine and chemokine production suggesting these hBDs link innate and adaptive immunity [5,31,32], we focused on correlating hBD levels with the cytokine/chemokine panel used in this current investigation. Results supported the hypothesis that there is a correlation between hBD’s and the immunoregulatory response.
At this stage, it is not clear why the levels of hBD-2 correlated only in asymptomatic, but not in symptomatic, irreversible pulpitis compared to the normal pulp (Table 3). In contrast, the levels of hBD-3 correlated in both ASIP and SIP (Table 4). These observations imply that there are differences between SIP and ASIP and the involvement of hBD’s and cytokines, chemokines. Studies are on-going in our laboratory to explore why SIP and ASIP may be different and the potential contribution of alternative inflammatory mediators such as neuropeptides and microbial differences between the two cases (SIP and ASIP groups).
The results of this study agree with Dommisch et al. who reported, in their in-vitro study, that hBD-2 stimulated the gene expression of pro-inflammatory cytokines [23]. Our findings also agree with Kim et al. [22] who reported that there was a correlation between TNF-α and hBD-2. The reason why, in the SIP group there was a correlation between the hBD-3 and cytokines, chemokines (Table 4) but not hBD-2 levels with the cytokines and the chemokines, needs further investigation (Table 3). In both SIP and ASIP groups (Table 4) there was a significant correlation between hBD-3 and all the chemokine and cytokines. Since the levels of hBD-3 were correlated in SIP and ASIP groups, this again might suggest that perhaps hBD-3 may play an even more extensive role in immunoregulation than previously reported in the endodontic literature.
There are many speculations and possibilities as to why an inflamed pulp might be symptomatic or asymptomatic. These possibilities include the presence of endotoxins [33], the immunoregulary response [34-36], and pathogens of various microbial progression [24,37-39]. The latter describes how various microbes elicit different immunologic responses. In the periodontal literature, hBD-3 was reported to bind to a strain of Porphyromonas gingivalis and attenuated a proinflammatory response [40]. The correlation between hBD-3 and the microbe was significantly higher than hBD1 or hBD-2 resulting in significant attenuation of the interleukin (IL)-6, IL-10, granulocyte macrophage colony stimulating factor (GM-CSF) and tumornecrosis factor-a (TNF-α).
Other similar studies [14,40 41] suggest that different microbial pathogens elicit different immunological responses. In future studies, differences between microbial pathogens in symptomatic and asymptomatic irreversible pulpitis should be better explored.
We do agree that the sample size for our normal group was smaller compared to the other two groups (SIP and ASIP). We would have preferred to have had a larger sample size for normal subjects, but it is not easy to get samples from teeth with normal pulps. However, the previous studies [8,42,43] had a similar sample size as ours for their control group and based on these previous studies we performed our power calculation of the study as detailed in the Methodology Section.
We used paper points for our clinical sampling technique. Previous clinical studies (excluding extraction of teeth) have used paper points [27,28,44-50], cotton pellets [34,51], barbed broach [43] to measure cytokines, chemokines, neuropeptides and exotoxins in the root canal system. Currently there are no “gold standard techniques” for sampling pulpal tissue, and to date, the current molecular-based methods are still under continuous improvement [52].
Future studies evaluating microbial differences in root canals and the concomitant host responses could provide an interesting contribution to the understanding of the host-pathogen relationship.
Conclusions
Human beta defensin-2 and hBD-3 were associated with the cytokines and chemokines in ASIP group. HBD-3 concentrations correlated with the levels of the chemokine and the cytokines in the SIP and ASIP groups.
Acknowledgement The authors deny any conflicts of interest.
References
1. Hahn CL, Liewehr FR. Update on the adaptive immune responses of the dental pulp. (2007) J Endod 33: 773-781.
2. Hahn CL, Liewehr FR. Innate immune responses of the dental pulp to caries. (2007) J Endod 33: 643-651.
3. Hahn CL, Liewehr FR. Relationships between caries bacteria, host responses, and clinical signs and symptoms of pulpitis. (2007) J Endod 33: 213-219.
4. Ganz T1. Defensins: antimicrobial peptides of innate immunity. (2003) Nat Rev Immunol 3: 710-720. 5. Yang D, Chertov O, Bykovskaia S, Chen Q, Buffo M, et al. ß-Defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. (1999) Science 286:525-528.
6. Dunsche A, Açil Y, Dommisch H, Siebert R, Schröder JM, et al.. The novel human beta-defensin-3 is widely expressed in oral tissues. (2002) Eur J Oral Sci 110: 121-124.
7. Shiba H, Mouri Y, Komatsuzawa H, Ouhara K, Takeda K, et al.. Macrophage inflammatory protein-3alpha and beta-defensin-2 stimulate dentin sialophosphoprotein gene expression in human pulp cells. (2003) Biochem Biophys Res Commun 306: 867-871.
8. Paris S, Wolgin M, Kielbassa AM, Pries A, Zakrzewicz A. Gene expression of human beta-defensins in healthy and inflamed human dental pulps. (2009) J Endod 35: 520-523.
9. Ghosh SK, Gerken TA, Schneider KM, Feng Z, McCormick TS, et al. Quantification of human ß-defensin-2 and-3 in body fluids: application for studies of innate immunity. (2007) Clin Chem 53:757-765.
10. Yadava P, Zhang C, Sun J, Hughes JA. Antimicrobial activities of human ß-defensins against Bacillus species. (2006) International Journal of antimicrobial agents 28:132-137.
11. Feng Z, Dubyak GR, Lederman MM, Weinberg A. Cutting edge: human beta defensin 3--a novel antagonist of the HIV-1 coreceptor CXCR4. (2006) J Immunol 177: 782-786.
12. Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, et al.. Defensins. Natural peptide antibiotics of human neutrophils. (1985) J Clin Invest 76: 1427-1435.
13. Soruri A, Grigat J, Forssmann U, Riggert J, Zwirner J. beta-Defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. (2007) Eur J Immunol 37: 2474-2486.
14. Kota S, Sabbah A, Harnack R, Xiang Y, Meng X, et al. Role of human ß-defensin-2 during tumor necrosis factor-a/NF-?B-mediated innate antiviral response against human respiratory syncytial virus. (2008) J Biol Chem 283: 22417-22429.
15. Krisanaprakornkit S, Kimball JR, Dale BA. Regulation of human ß-defensin-2 in gingival epithelial cells: the involvement of mitogen-activated protein kinase pathways, but not the NF-?B transcription factor family. (2002) The Journal of Immunology168: 316-324.
16. Boughan PK, Argent RH, Body-Malapel M, Park J-H, Ewings KE, et al. Nucleotide-binding Oligomerization Domain-1 and Epidermal Growth Factor Receptor critical regulators of ß-defensins during helicobacter pylori infection.(2006) J Biol Chem 281: 11637-11648.
17. Sørensen OE, Thapa DR, Rosenthal A, Liu L, Roberts AA, et al. Differential regulation of ß-defensin expression in human skin by microbial stimuli. (2005) J Immunol. 174: 4870-4879.
18. Tiriveedhi V, Banan B, Deepti S, Nataraju A, Hachem R, et al.. Role of defensins in the pathogenesis of chronic lung allograft rejection. (2014) Hum Immunol 75: 370-377.
19. Guo B, Xie G, Duan Z, Xia L. The effects of recombinant human betadefensin-3 on expression of interleukin-17A and interleukin-22 in BEAS-2B cell. (2013) Chinese journal of experimental and clinical virology 27: 260-262.
20. Kanda N, Kamata M, Tada Y, Ishikawa T, Sato S, et al.. Human β-defensin-2 enhances IFN-γ and IL-10 production and suppresses IL-17 production in T cells. (2011) J Leukoc Biol 89: 935-944.
21. Petrov V, Funderburg N, Weinberg A, Sieg S. Human ß defensin-3 induces chemokines from monocytes and macrophages: diminished activity in cells from HIV-infected persons. (2013) Immunology 140: 413-420.
22. Kim YS, Min KS, Lee SI, Shin SJ, Shin KS, et al. Effect of proinflammatory cytokines on the expression and regulation of human beta-defensin 2 in human dental pulp cells. (2010) J Endod 36: 64-69.
23. Dommisch H, Winter J, Willebrand C, Eberhard J, Jepsen S. Immune regulatory functions of human beta-defensin-2 in odontoblast-like cells. (2007) Int Endod J 40: 300-307.
24. Hahn CL, Liewehr FR. Relationships between caries bacteria, host responses, and clinical signs and symptoms of pulpitis. (2007) J Endod 33: 213-219.
25. Levin LG, Law AS, Holland GR, Abbott PV, Roda RS. Identify and define all diagnostic terms for pulpal health and disease states. (2009) J Endod 35: 1645-1657.
26. AAE. Glossary of Endodontic Terms. (2011) [cited 071315].
27. Martinho FC, Gomes BP. Quantification of endotoxins and cultivable bacteria in root canal infection before and after chemomechanical preparation with 2.5% sodium hypochlorite. (2008) J Endod 34: 268-272.
28. Martinho FC, Nascimento GG, Leite FR, Gomes AP, Freitas LF, et al. Clinical Influence of Different Intracanal Medications on Th1-type and Th2type Cytokine Responses in Apical Periodontitis. (2015) J Endod 41:169-175.
29. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, et al. Measurement of protein using bicinchoninic acid. (1985) Anal Biochem 150: 76-85.
30. Walker JM. The bicinchoninic acid (BCA) assay for protein quantitation. In: The Protein Protocols Handbook. Springer. (2009) Pp:11-15.
31. Klüver E, Schulz-Maronde S, Scheid S, Meyer B, Forssmann WG, et al. Structure-activity relation of human ß-defensin 3: influence of disulfide bonds and cysteine substitution on antimicrobial activity and cytotoxicity(2005) Biochemistry 44: 9804-9816.
32. Funderburg N, Lederman MM, Feng Z, Drage MG, Jadlowsky J, et al. Human ß-defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. (2007) Proc Natl Acad Sci U S A104:1863118635.
33. Khabbaz MG, Anastasiadis PL, Sykaras SN. Determination of endotoxins in caries: association with pulpal pain. (2000) Int Endod J 33: 132-137.
34. Elsalhy M, Azizieh F, Raghupathy R. Cytokines as diagnostic markers of pulpal inflammation. (2013) Int Endod J 46: 573-580.
35. Cohen JS, Reader A, Fertel R, Beck M, Meyers WJ. A radioimmunoassay determination of the concentrations of prostaglandins E2 and F2alpha in painful and asymptomatic human dental pulps. (1985) J Endod 11: 330-335.
36. Byers MR. Dynamic plasticity of dental sensory nerve structure and cytochemistry. (1994) Arch Oral Biol 39 Suppl: 13S-21S.
37. Hahn CL, Best AM, Tew JG. Comparison of type 1 and type 2 cytokine production by mononuclear cells cultured with streptococcus mutans and selected other caries bacteria. (2004) J Endod 30: 333-338.
38. Gomes BP, Drucker DB, Lilley JD. Associations of specific bacteria with some endodontic signs and symptoms. (1994) Int Endod J 27: 291-298.
39. Jacinto R, Gomes B, Ferraz C, Zaia A, FJ Filho S. Microbiological analysis of infected root canals from symptomatic and asymptomatic teeth with periapical periodontitis and the antimicrobial susceptibility of some isolated anaerobic bacteria. (2003) Oral Microbiol Immunol 18:285-292.
40. Pingel LC, Kohlgraf KG, Hansen CJ, Eastman CG, Dietrich DE, et al. Human ß-defensin 3 binds to hemagglutinin B (rHagB), a non-fimbrial adhesin from Porphyromonas gingivalis, and attenuates a pro-inflammatory cytokine response. (2008) Immunol Cell Biol. 86: 643-649.
41. Varoga D, Tohidnezhad M, Paulsen F, Wruck CJ, Brandenburg L, et al.. The role of human beta-defensin-2 in bone. (2008) J Anat 213: 749-757.
42. Abd-Elmeguid A, Abdeldayem M, Kline LW, Moqbel R, Vliagoftis H, et al.. Osteocalcin expression in pulp inflammation. (2013) J Endod 39: 865-872.
43. Kokkas A, Goulas A, Varsamidis K, Mirtsou V, Tziafas D. Irreversible but not reversible pulpitis is associated with up-regulation of tumour necrosis factoralpha gene expression in human pulp. (2007) Int Endod J 40:198-203.
44. Safavi KE, Rossomando EF. Tumor necrosis factor identified in periapical tissue exudates of teeth with apical periodontitis. (1991) J Endod 17: 12-14.
45. Martinho FC, Chiesa WM, Leite FR, Cirelli JA, Gomes BP. Correlation between clinical/radiographic features and inflammatory cytokine networks produced by macrophages stimulated with endodontic content. (2012) J Endod 38:740-745.
46. Martinho FC, Chiesa WMM, Leite FR, Cirelli JA, Gomes BP. Antigenic Activity of Bacterial Endodontic Contents from Primary Root Canal Infection with Periapical Lesions against Macrophage in the Release of Interleukin-1ß and Tumor Necrosis Factor alpha. (2010) J Endod 36:1467-1474.
47. Martinho FC, Chiesa WMM, Leite FR, Cirelli JA, Gomes BP. Antigenicity of Primary Endodontic Infection against Macrophages by the Levels of PGE 2 Production. (2011) J Endod 37: 602-607.
48. Martinho FC, Chiesa WM, Zaia AA, Ferraz CC, Almeida JF, et al.. Comparison of endotoxin levels in previous studies on primary endodontic infections. (2011) J Endod 37: 163-167.
49. Sousa EL, Martinho FC, Leite FR, Nascimento GG, Gomes BP. Macrophage cell activation with acute apical abscess contents determined by interleukin-1 Beta and tumor necrosis factor alpha production. (2014) J Endod 40: 1752-1757.
50. Yoo YJ, Shon WJ, Baek SH, Kang MK, Kim HC, et al.. Effect of 1440-nanometer neodymium:yttrium-aluminum-garnet laser irradiation on pain and neuropeptide reduction: a randomized prospective clinical trial. (2014) J Endod 40: 28-32.
51. Nakanishi T, Matsuo T, Ebisu S. Quantitative analysis of immunoglobulins and inflammatory factors in human pulpal blood from exposed pulps. (1995) J Endod 21: 131-136.
52. Sathorn C, Parashos P, Messer HH. How useful is root canal culturing in predicting treatment outcome? (2007) J Endod 33: 220-225.
Keywords


 PDF
PDF